
Assertion-
A special line will be seen for $2{{p}_{x}}-2{{p}_{y}}$ transition.
Reason-
Energy is released in the form of wave of light when the ${{e}^{-}}$ drops from $2{{p}_{x}}$ to $2{{p}_{y}}$ orbital.
A) Both assertion and reason are correct and reason is the correct explanation for assertion.
B) Both assertion and reason are correct but reason is not the correct explanation for assertion.
C) Assertion is correct but reason is incorrect
D) Both assertion and reason are incorrect
Answer
561.3k+ views
Hint: The energies of both the orbitals should be considered, and the orientation also must be taken into account.
- Spectral lines, dark or bright lights appear as a result of absorption or emission of energy or light, a source of energy in a narrow frequency.
Complete step by step answer:
- So the question consists of two parts: an assertion sentence and a reason which supports the assertion sentence.
The question is all about the 2 p orbitals, its transitions and the spectral lines related to the transitions.
So let’s decode the sentences and find the correct option, for that we should know about the basic idea orbitals and the number of orbitals in p subshell, its orientation and energy.
-So what’s an orbital?
An orbital is a region near the nucleus where we have the greatest probability of finding electrons.
- Now the question is how much orbitals in the p subshell, its orientation and energy. By getting the answers for these questions it’s easy for us to solve the puzzle.
P-orbital is having an azimuthal or angular quantum number of value 2.
To find the number of orbitals from azimuthal or angular quantum number, the equation is,
Number of orbitals = 2l + 1, where l is the value of azimuthal quantum number.
Number of orbitals = 2(1) + 1 = 3
So the number of orbitals is three. The p orbitals are dumbbell shaped structures oriented in each axis.
If the orbital is oriented in x –axis then it is called as ${{p}_{x}}$ orbital
If it is oriented in y-axis, then it is called as ${{p}_{y}}$ orbital
And if in the z-axis, it is called as ${{p}_{z}}$ orbital.
So the three orbitals are ${{p}_{x}},{{p}_{y}},{{p}_{z}}$
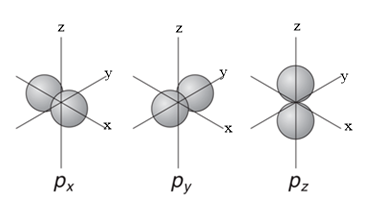
- Now if we talk about the energy levels, the three orbitals are degenerate i.e. all the three orbitals have the same energy level.
- So if the electrons move from ${{p}_{x-}}{{p}_{y}},{{p}_{y}}-{{p}_{z}}$ and ${{p}_{x-}}{{p}_{z}}$, no energy is absorbed or emitted. So if no absorption or emission of energy in the form of light say photons involved in a transition then we will not observe any spectral lines.
- So we can conclude that transitions involved in p-orbitals will not produce spectral lines, since the p-orbitals are degenerate.
So both the assertion and reason given is incorrect. The correct option is option “D” .
Note: Always study about the orientation, energy and orbital splitting nature of orbitals if the question is about the spectral lines and its problems. And find whether the electrons in the orbitals absorb or emit the energy source to determine which type of spectral lines we will observe.
- Spectral lines, dark or bright lights appear as a result of absorption or emission of energy or light, a source of energy in a narrow frequency.
Complete step by step answer:
- So the question consists of two parts: an assertion sentence and a reason which supports the assertion sentence.
The question is all about the 2 p orbitals, its transitions and the spectral lines related to the transitions.
So let’s decode the sentences and find the correct option, for that we should know about the basic idea orbitals and the number of orbitals in p subshell, its orientation and energy.
-So what’s an orbital?
An orbital is a region near the nucleus where we have the greatest probability of finding electrons.
- Now the question is how much orbitals in the p subshell, its orientation and energy. By getting the answers for these questions it’s easy for us to solve the puzzle.
P-orbital is having an azimuthal or angular quantum number of value 2.
To find the number of orbitals from azimuthal or angular quantum number, the equation is,
Number of orbitals = 2l + 1, where l is the value of azimuthal quantum number.
Number of orbitals = 2(1) + 1 = 3
So the number of orbitals is three. The p orbitals are dumbbell shaped structures oriented in each axis.
If the orbital is oriented in x –axis then it is called as ${{p}_{x}}$ orbital
If it is oriented in y-axis, then it is called as ${{p}_{y}}$ orbital
And if in the z-axis, it is called as ${{p}_{z}}$ orbital.
So the three orbitals are ${{p}_{x}},{{p}_{y}},{{p}_{z}}$

- Now if we talk about the energy levels, the three orbitals are degenerate i.e. all the three orbitals have the same energy level.
- So if the electrons move from ${{p}_{x-}}{{p}_{y}},{{p}_{y}}-{{p}_{z}}$ and ${{p}_{x-}}{{p}_{z}}$, no energy is absorbed or emitted. So if no absorption or emission of energy in the form of light say photons involved in a transition then we will not observe any spectral lines.
- So we can conclude that transitions involved in p-orbitals will not produce spectral lines, since the p-orbitals are degenerate.
So both the assertion and reason given is incorrect. The correct option is option “D” .
Note: Always study about the orientation, energy and orbital splitting nature of orbitals if the question is about the spectral lines and its problems. And find whether the electrons in the orbitals absorb or emit the energy source to determine which type of spectral lines we will observe.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




