
Bakelite is formed by the reaction of:
A. Phenol and formaldehyde
B. Formaldehyde and aniline
C. Adipic acid and ethylene glycol
D. Phthalic acid and ethylene glycol.
Answer
588k+ views
Hint: The materials made up from the long chains of the molecules are known as polymers and the process used to make them is known as polymerisation. Bakelite is also a polymer made up from the synthetic components, it comes under the category of plastic.
Complete step by step answer: Bakelite is basically a polymer made up of synthetic components. The monomer units used in making Bakelite are phenol and formaldehyde.
3d Structure of Bakelite is:
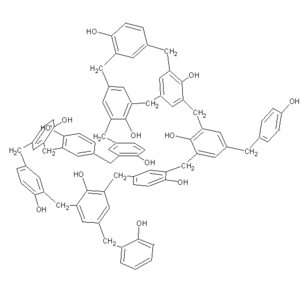
Bakelite is formed from the reaction of phenol and formaldehyde. Basically it is formed in two steps. In the first step formaldehyde reacts with phenol and produces o-Hydroxymethyl phenol. Then o-Hydroxy methyl phenol and phenol come together and form Bakelite.The reaction is condensation polymerisation as two molecules condense and give out by-product. In case of bakelite phenol and formaldehyde condense to form the monomer giving out $H_2O$ as a by-product. Bakelite is also called a co-polymer.
Reaction:
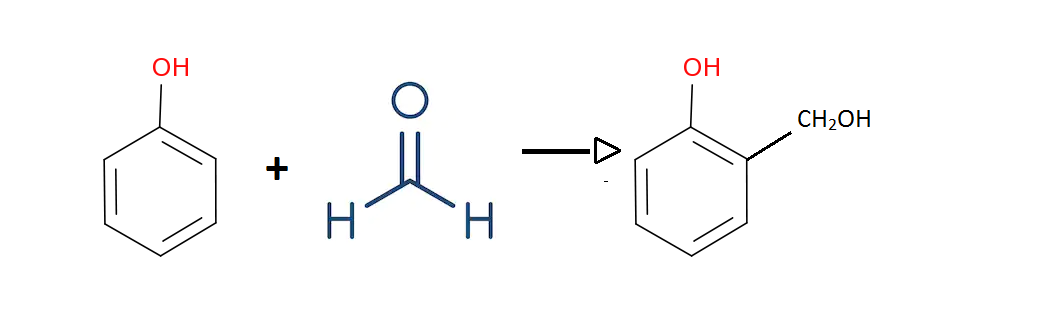
Phenol reacts with formaldehyde to produce o-Hydroxy methyl phenol.
Now produced complex (o-Hydroxymethyl phenol) and phenol come together to form Bakelite
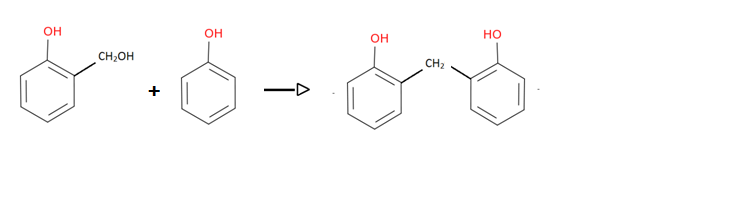
Hence the obtained product in the above reaction is Bakelite.
And when this reaction proceeds at larger extent or we can say multiple units come together, Bakelite will expand its size gradually and make a chain like structure.
Hence we can say that Bakelite is formed by the reaction of Phenol and formaldehyde.
So, the correct answer is “Option A”.
Note:
Bakelite is widely used in our daily life. It has high resistance to heat and electricity both so it is used in electrical appliances and in making handles of utensils. Due to high tensile strength it has some industrial uses also.
Complete step by step answer: Bakelite is basically a polymer made up of synthetic components. The monomer units used in making Bakelite are phenol and formaldehyde.
3d Structure of Bakelite is:

Bakelite is formed from the reaction of phenol and formaldehyde. Basically it is formed in two steps. In the first step formaldehyde reacts with phenol and produces o-Hydroxymethyl phenol. Then o-Hydroxy methyl phenol and phenol come together and form Bakelite.The reaction is condensation polymerisation as two molecules condense and give out by-product. In case of bakelite phenol and formaldehyde condense to form the monomer giving out $H_2O$ as a by-product. Bakelite is also called a co-polymer.
Reaction:
Phenol reacts with formaldehyde to produce o-Hydroxy methyl phenol.

Now produced complex (o-Hydroxymethyl phenol) and phenol come together to form Bakelite

Hence the obtained product in the above reaction is Bakelite.
And when this reaction proceeds at larger extent or we can say multiple units come together, Bakelite will expand its size gradually and make a chain like structure.
Hence we can say that Bakelite is formed by the reaction of Phenol and formaldehyde.
So, the correct answer is “Option A”.
Note:
Bakelite is widely used in our daily life. It has high resistance to heat and electricity both so it is used in electrical appliances and in making handles of utensils. Due to high tensile strength it has some industrial uses also.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




