
Benzene is passed through \[Na\] in \[N{H_3}\] solution then the major product was passed through ozone and \[Zn - {H_2}O\] solution then the product of it was passed through \[Zn - Hg\] in \[Conc.HCl\] solution to give a product. What and how is the product formed?
Answer
495.3k+ views
Hint: Benzene is an aromatic compound and when reacted with sodium in ammonia undergo reduction to form \[1,4\] cyclohexadiene. When this compound passes through ozone and \[Zn - {H_2}O\] solution forms an aldehyde compound. When aldehyde is passed through \[Zn - Hg\] in \[Conc.HCl\] solution forms propane.
Complete answer:
Given that benzene is passed through \[Na\] in \[N{H_3}\] solution. The reaction is named as Birch reduction. Aromatic compound when passed through \[Na\] in \[N{H_3}\] solution undergoes reduction to form a \[1,4\] cyclohexadiene.
The chemical reaction involved will be as follows-
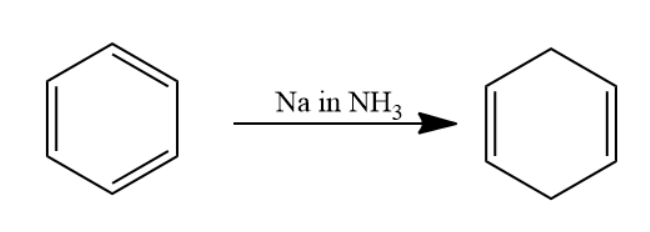
When this \[1,4\] cyclohexadiene is passed through ozone and \[Zn - {H_2}O\] solution the double bond will converts into carbonyl group forms two molecules of dialdehyde compound named as \[1,3 - propane - di - al\]. Two moles of dialdehyde compound form in this reaction.
The chemical reaction involved will be as follows-
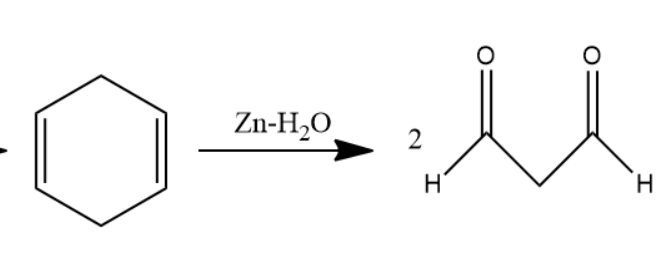
Later, \[1,3 - propane - di - al\] passed through \[Zn - Hg\] in \[Conc.HCl\] solution undergoes clemmensen reduction to form alkanes. Carbonyl group converts into alkane in clemmensen reduction. Two molecules of \[1,3 - propane - di - al\] convert into two molecules of propane.
The chemical reaction involved will be as follows:
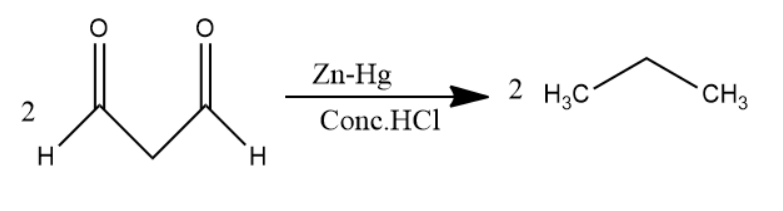
Thus, Benzene is passed through \[Na\] in \[N{H_3}\] solution then the major product was passed through ozone and \[Zn - {H_2}O\] solution then the product of it was passed through \[Zn - Hg\] in \[Conc.HCl\] solution to give a product. The product is propane.
Note:
Birch reduction can occur in two types based on the group present on the aromatic ring. When there is a presence of an electron releasing group then the reduction takes place at \[2,5\] positions. When there is a presence of an electron withdrawing group then the reduction takes place at \[1,4\] positions.
Complete answer:
Given that benzene is passed through \[Na\] in \[N{H_3}\] solution. The reaction is named as Birch reduction. Aromatic compound when passed through \[Na\] in \[N{H_3}\] solution undergoes reduction to form a \[1,4\] cyclohexadiene.
The chemical reaction involved will be as follows-

When this \[1,4\] cyclohexadiene is passed through ozone and \[Zn - {H_2}O\] solution the double bond will converts into carbonyl group forms two molecules of dialdehyde compound named as \[1,3 - propane - di - al\]. Two moles of dialdehyde compound form in this reaction.
The chemical reaction involved will be as follows-

Later, \[1,3 - propane - di - al\] passed through \[Zn - Hg\] in \[Conc.HCl\] solution undergoes clemmensen reduction to form alkanes. Carbonyl group converts into alkane in clemmensen reduction. Two molecules of \[1,3 - propane - di - al\] convert into two molecules of propane.
The chemical reaction involved will be as follows:

Thus, Benzene is passed through \[Na\] in \[N{H_3}\] solution then the major product was passed through ozone and \[Zn - {H_2}O\] solution then the product of it was passed through \[Zn - Hg\] in \[Conc.HCl\] solution to give a product. The product is propane.
Note:
Birch reduction can occur in two types based on the group present on the aromatic ring. When there is a presence of an electron releasing group then the reduction takes place at \[2,5\] positions. When there is a presence of an electron withdrawing group then the reduction takes place at \[1,4\] positions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




