
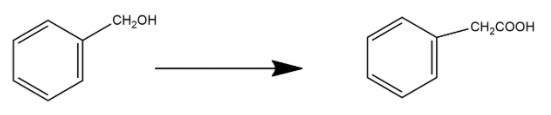
Benzyl alcohol to 2-phenyl ethanoic acid:

Answer
573.3k+ views
Hint: Alcohol are those which have $OH$ group present in them and they ended with the suffix ol and acids are those which have $COOH$ group and ended with the suffix oic acid. Aromatic compounds are those which have rings present in them.
Complete step by step answer:
Benzyl alcohol is an aromatic alcohol, it is a colorless liquid with mild pleasant aromatic odor. It is produced naturally by many plants and is commonly found in fruits and teas. Benzyl alcohol is converted into 2-phenyl ethanoic acid by following a step by step mechanism. 2-phenyl ethanoic acid also known by the name phenylacetic acid it is an organic compound which contains a phenyl functional group and a carboxylic functional group. It is generally a white solid having honey-like odor.
In first step benzyl alcohol gets reacted with $PC{{l}_{5}}$ and it forms benzoyl chloride with the loss of $POC{{l}_{3}}$ and $HCl$.
In the second step benzoyl chloride is reacted with $KCN$ or aqueous ethanol and undergoes nucleophilic substitution reaction and forms benzyl cyanide with the loss of $KCl$.
After this benzyl cyanide undergoes the process of hydrolysis and forms the final product i.e. 2-phenyl ethanoic acid.
The mechanism can be shown as follows:

In this way Benzyl alcohol gets converted into 2-phenyl ethanoic acid.
Note: Benzyl alcohol is used as a general solvent for inks, waxes, shellacs, paints, lacquers, and epoxy resin coatings. Phenylacetic acid is used in some perfumes due to its honey like odor even in low concentrations. It is also used in penicillin G production and diclofenac production.
Complete step by step answer:
Benzyl alcohol is an aromatic alcohol, it is a colorless liquid with mild pleasant aromatic odor. It is produced naturally by many plants and is commonly found in fruits and teas. Benzyl alcohol is converted into 2-phenyl ethanoic acid by following a step by step mechanism. 2-phenyl ethanoic acid also known by the name phenylacetic acid it is an organic compound which contains a phenyl functional group and a carboxylic functional group. It is generally a white solid having honey-like odor.
In first step benzyl alcohol gets reacted with $PC{{l}_{5}}$ and it forms benzoyl chloride with the loss of $POC{{l}_{3}}$ and $HCl$.
In the second step benzoyl chloride is reacted with $KCN$ or aqueous ethanol and undergoes nucleophilic substitution reaction and forms benzyl cyanide with the loss of $KCl$.
After this benzyl cyanide undergoes the process of hydrolysis and forms the final product i.e. 2-phenyl ethanoic acid.
The mechanism can be shown as follows:

In this way Benzyl alcohol gets converted into 2-phenyl ethanoic acid.
Note: Benzyl alcohol is used as a general solvent for inks, waxes, shellacs, paints, lacquers, and epoxy resin coatings. Phenylacetic acid is used in some perfumes due to its honey like odor even in low concentrations. It is also used in penicillin G production and diclofenac production.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




