
Chlorobenzene on fusing with solid $NaOH$ (at $623K$ and $320atm$ pressure) gives:
A. benzene
B. Benzoic acid
C. phenol
D. benzene chloride
Answer
501.3k+ views
Hint: When it comes to nucleophilic substitution reactions, chlorobenzene is extremely unreactive. This causes the electrons in the $C - Cl$ bond to delocalize, and a partial double bond character develops in the bond, making it difficult for the nucleophile to cleave it.
Complete answer:
When chlorobenzene is fused with solid $NaOH$ , it gives phenol as a product. This is a nucleophilic substitution reaction of chlorobenzene. Here hydroxide ion replaces chlorine ion from chlorobenzene and gives phenol as a product.
The reaction is as follows:
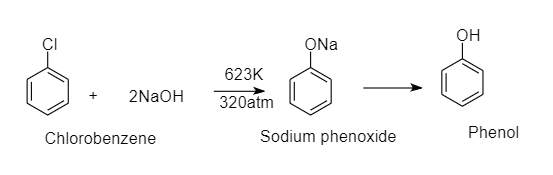
This reaction requires high pressure and temperature since chlorobenzene does not undergo nucleophilic substitution reactions easily.
A nucleophilic substitution reaction is one in which one nucleophile substitutes another in an organic process. It's very similar to normal displacement reactions in chemistry, in which a more reactive element replaces a less reactive element in a salt solution.
Hence, the correct option is C. phenol
Additional Information: The nucleophilicity of a nucleophile is its reactivity or strength in nucleophilic substitution processes. A stronger nucleophile replaces a weaker nucleophile from its component in a nucleophilic substitution process.
Note:
Sodium hydroxide is a white crystalline odourless substance that absorbs moisture from the air at ambient temperature. It's a synthetic chemical. When dissolved in water or neutralised with acid, it releases a significant amount of heat, which could ignite combustible objects. Sodium hydroxide is a highly corrosive substance. It's usually applied as a solid or a $50\% $ solution.
Complete answer:
When chlorobenzene is fused with solid $NaOH$ , it gives phenol as a product. This is a nucleophilic substitution reaction of chlorobenzene. Here hydroxide ion replaces chlorine ion from chlorobenzene and gives phenol as a product.
The reaction is as follows:

This reaction requires high pressure and temperature since chlorobenzene does not undergo nucleophilic substitution reactions easily.
A nucleophilic substitution reaction is one in which one nucleophile substitutes another in an organic process. It's very similar to normal displacement reactions in chemistry, in which a more reactive element replaces a less reactive element in a salt solution.
Hence, the correct option is C. phenol
Additional Information: The nucleophilicity of a nucleophile is its reactivity or strength in nucleophilic substitution processes. A stronger nucleophile replaces a weaker nucleophile from its component in a nucleophilic substitution process.
Note:
Sodium hydroxide is a white crystalline odourless substance that absorbs moisture from the air at ambient temperature. It's a synthetic chemical. When dissolved in water or neutralised with acid, it releases a significant amount of heat, which could ignite combustible objects. Sodium hydroxide is a highly corrosive substance. It's usually applied as a solid or a $50\% $ solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




