
$CO_{3}^{2-}$ anions has which of the following characteristics?
This question has multiple correct options
A. bonds of unequal length
B. $s{{p}^{2}}$ hybridization of C atom
C. resonance stabilization
D. same bond angles
Answer
573.6k+ views
Hint: As we know that $CO_{3}^{2-}$ is called carbonate ion. It is the simplest oxocarbon anion, that has one carbon atom which is surrounded by three oxygen atoms. It is found that $CO_{3}^{2-}$ is a polyatomic ion.
Complete answer:
- Let’s discuss about bond lengths of $CO_{3}^{2-}$ ions:
It is found that there are three identical bonds present in $CO_{3}^{2-}$. We can represent the Lewis structure of $CO_{3}^{2-}$ as:
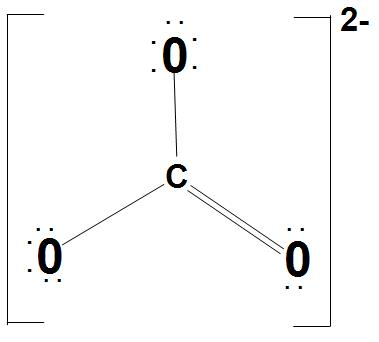
- Let’s discuss about $s{{p}^{2}}$ hybridization of C atom:
It is found that the carbonate ion, carbon is bonded with one oxygen atom by a double bond and with two oxygen by a single bond. It has trigonal planar geometry which clearly mentions that the carbon is $s{{p}^{2}}$ hybridized.
- Let’s discuss resonance stabilization: It is found that the carbonate anion shows resonance stabilization. We can see the resonance structures of carbonate ion with resonance hybrid:
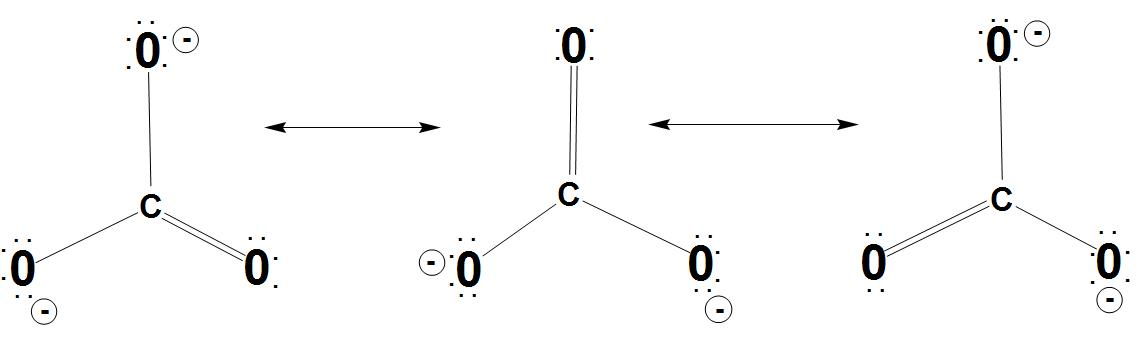
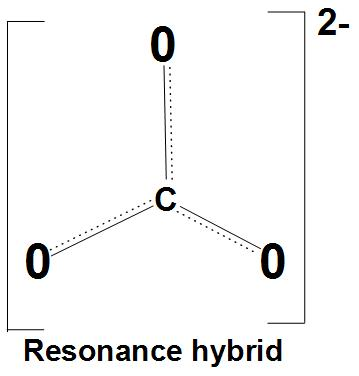
- Let’s discuss about same bond angles:
As we have discussed , the carbonate ion has trigonal planar geometry, just like $B{{F}_{3}}$ , with a bond angle of 120 degree. It is found that carbonate ions have the same bond angles.
- Hence, we can conclude that the correct options are (b), (c), (d) that is $CO_{3}^{2-}$ has $s{{p}^{2}}$ hybridization of C atom, resonance stabilization and same bond angles.
Note: - $CO_{3}^{2-}$ should not be confused with $C{{O}_{3}}$. The main difference between both of these is that $CO_{3}^{2-}$ is carbonate ion, which is a stable oxocarbon anion. Whereas, $C{{O}_{3}}$ is carbon trioxide, which is an unstable oxide of carbon (oxocarbon) .
Complete answer:
- Let’s discuss about bond lengths of $CO_{3}^{2-}$ ions:
It is found that there are three identical bonds present in $CO_{3}^{2-}$. We can represent the Lewis structure of $CO_{3}^{2-}$ as:

- Let’s discuss about $s{{p}^{2}}$ hybridization of C atom:
It is found that the carbonate ion, carbon is bonded with one oxygen atom by a double bond and with two oxygen by a single bond. It has trigonal planar geometry which clearly mentions that the carbon is $s{{p}^{2}}$ hybridized.
- Let’s discuss resonance stabilization: It is found that the carbonate anion shows resonance stabilization. We can see the resonance structures of carbonate ion with resonance hybrid:


- Let’s discuss about same bond angles:
As we have discussed , the carbonate ion has trigonal planar geometry, just like $B{{F}_{3}}$ , with a bond angle of 120 degree. It is found that carbonate ions have the same bond angles.
- Hence, we can conclude that the correct options are (b), (c), (d) that is $CO_{3}^{2-}$ has $s{{p}^{2}}$ hybridization of C atom, resonance stabilization and same bond angles.
Note: - $CO_{3}^{2-}$ should not be confused with $C{{O}_{3}}$. The main difference between both of these is that $CO_{3}^{2-}$ is carbonate ion, which is a stable oxocarbon anion. Whereas, $C{{O}_{3}}$ is carbon trioxide, which is an unstable oxide of carbon (oxocarbon) .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




