
$CsBr$ crystallizes in a body-centered cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of \[Cs = 133\] and that of $Br = 80$ amu and Avogadro number being $6.02 \times {10^{23}}mo{l^{ - 1}}$ , the density of $CsBr$ is:
A.$42.5g/c{m^3}$
B.$0.425g/c{m^3}$
C.$8.5g/c{m^3}$
D.$4.25g/c{m^3}$
Answer
584.4k+ views
Hint:
Lattice structures are topologically ordered, three-dimensional open-celled structures composed of one or more repeating unit cells [2,3]. These cells are defined by the dimensions and connectivity of their constituent strut elements, which are connected at specific nodes.This question can be solved by using the formula $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ where $Z,M,a,{N_A},\rho $ are number of atoms, Mass of consisting atom/molecule, edge length/unit cell length, Avogadro number and density respectively.
Value of $Z$ for BCC is 2.
Complete step by step answer:
Let’s deduce the expression $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ from the very beginning.
We know density of unit cell =mass of unit cell/volume of unit cell
Mass of a unit cell = number of atoms in unit cell $ \times $ mass of single atom = $Z \times m - - - (1)$ where, $Z,m$ are number of atoms and mass of single atom/molecule in unit cell. We can find the mass of a single atom/molecular by dividing atomic mass $M$ by Avogadro’s Number ${N_A}$.
So, $m = \dfrac{M}{{{N_A}}} - - - (2)$
From equation (1) & (2)
Mass of unit cell $ = \dfrac{{Z \times M}}{{{N_A}}} - - - (3)$
Now we can write density of unit cell $\rho = \dfrac{{Z \times M}}{{V \times {N_A}}} - - - (4)$ where $V$ is volume of unit cell. As we also know that the volume of a cube is cube of its edge length, consider an edge length $a$ , so volume $V = {a^3}$.
So, equation (4) can be rewritten as $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}} - - - (5)$ .
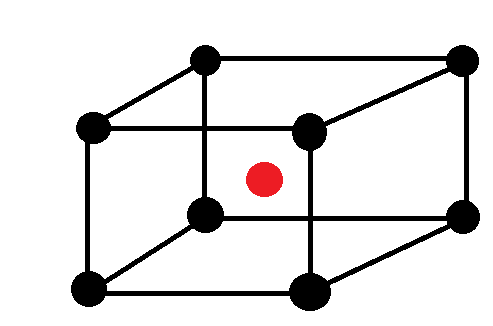
Now, Let’s calculate the number of atoms in the unit cell that is BCC.
Atoms at the corners are 8 and contribution of each corner atom is 1/8 and atom at centre is one and it is not shared with any other cell.
So, $Z = 8 \times \dfrac{1}{8} + 1 = 2$
As per question $a = 436.6pm = 436.6 \times {10^{ - 10}}cm,M = 133 + 80 = 213g/mol$
Putting all values in equation (5) we get,
$\rho = \dfrac{{2 \times 213g/mol}}{{{{(436.6 \times {{10}^{ - 10}}cm)}^3} \times 6.02 \times {{10}^{23}}mo{l^{ - 1}}}}$
$ \Rightarrow \rho = 8.5g/c{m^3}$
Note:
While deducing the formula $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ we used $m$ and $M$ , both are different. $m$ is mass of single atom $M$ is atomic mass in amu. So, when we take $M$ atomic mass in grams, according to concept of moles, we get mass of 1 mole of atoms that are equal in Avogadro’s number $ = 6.02 \times {10^{23}}mo{l^{ - 1}}$ . So, in order to find the mass of a single atom $m = \dfrac{M}{{{N_A}}}$ .
Lattice structures are topologically ordered, three-dimensional open-celled structures composed of one or more repeating unit cells [2,3]. These cells are defined by the dimensions and connectivity of their constituent strut elements, which are connected at specific nodes.This question can be solved by using the formula $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ where $Z,M,a,{N_A},\rho $ are number of atoms, Mass of consisting atom/molecule, edge length/unit cell length, Avogadro number and density respectively.
Value of $Z$ for BCC is 2.
Complete step by step answer:
Let’s deduce the expression $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ from the very beginning.
We know density of unit cell =mass of unit cell/volume of unit cell
Mass of a unit cell = number of atoms in unit cell $ \times $ mass of single atom = $Z \times m - - - (1)$ where, $Z,m$ are number of atoms and mass of single atom/molecule in unit cell. We can find the mass of a single atom/molecular by dividing atomic mass $M$ by Avogadro’s Number ${N_A}$.
So, $m = \dfrac{M}{{{N_A}}} - - - (2)$
From equation (1) & (2)
Mass of unit cell $ = \dfrac{{Z \times M}}{{{N_A}}} - - - (3)$
Now we can write density of unit cell $\rho = \dfrac{{Z \times M}}{{V \times {N_A}}} - - - (4)$ where $V$ is volume of unit cell. As we also know that the volume of a cube is cube of its edge length, consider an edge length $a$ , so volume $V = {a^3}$.
So, equation (4) can be rewritten as $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}} - - - (5)$ .

Now, Let’s calculate the number of atoms in the unit cell that is BCC.
Atoms at the corners are 8 and contribution of each corner atom is 1/8 and atom at centre is one and it is not shared with any other cell.
So, $Z = 8 \times \dfrac{1}{8} + 1 = 2$
As per question $a = 436.6pm = 436.6 \times {10^{ - 10}}cm,M = 133 + 80 = 213g/mol$
Putting all values in equation (5) we get,
$\rho = \dfrac{{2 \times 213g/mol}}{{{{(436.6 \times {{10}^{ - 10}}cm)}^3} \times 6.02 \times {{10}^{23}}mo{l^{ - 1}}}}$
$ \Rightarrow \rho = 8.5g/c{m^3}$
Note:
While deducing the formula $\rho = \dfrac{{Z \times M}}{{{a^3} \times {N_A}}}$ we used $m$ and $M$ , both are different. $m$ is mass of single atom $M$ is atomic mass in amu. So, when we take $M$ atomic mass in grams, according to concept of moles, we get mass of 1 mole of atoms that are equal in Avogadro’s number $ = 6.02 \times {10^{23}}mo{l^{ - 1}}$ . So, in order to find the mass of a single atom $m = \dfrac{M}{{{N_A}}}$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




