
Cyanohydrin of which compound on hydrolysis will give lactic acid?
A. \[{{\rm{C}}_6}{{\rm{H}}_5}{\rm{CHO}}\]
B. \[{\rm{HCHO}}\]
C. \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\]
D. \[{\rm{C}}{{\rm{H}}_3}{\rm{C}}{{\rm{H}}_2}{\rm{CHO}}\]
Answer
567k+ views
Hint: Lactic acid contains three carbon atoms. It is obtained from the cyanohydrin of an aldehyde. The number of carbon atoms in the cyanohydrin will be the same as the number of carbon atoms in lactic acid. But the starting aldehyde will have one less carbon atom, as one carbon comes from the cyano group.
Complete step by step answer:
The aldehydes react with hydrogen cyanide to form cyanohydrin.

In the above reaction, ‘R’ represents an alkyl group. The cyanohydrin has an additional carbon atom than aldehyde. This additional carbon atom comes from hydrogen cyanide. The cyano group of hydrogen cyanide is added to carbonyl carbon and the hydrogen atom of hydrogen cyanide is added to carbonyl oxygen. A new carbon-carbon single bond is formed and the carbon-oxygen double bond is converted into a single bond. Aldehyde contains carbonyl groups whereas cyanohydrin contains one hydroxyl group and one cyano group.
The hydrolysis of cyanohydrin gives alpha hydroxy, carboxylic acids.
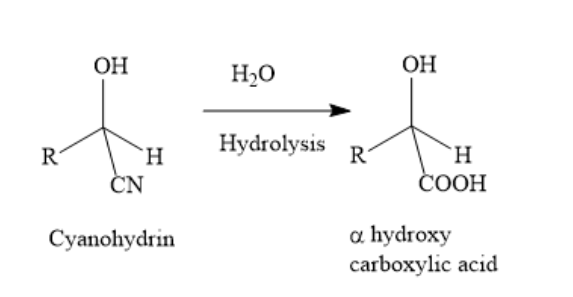
In the above reaction, the cyano group is hydrolyzed to a carboxylic acid functional group. The number of carbon atoms in the alpha hydroxy carboxylic acid is the same as the number of carbon atoms in the cyanohydrin.
When an alpha hydroxy carboxylic acid is given and it is asked to determine the structure of aldehyde, whose cyanohydrin upon hydrolysis will give the given alpha hydroxy carboxylic acid, then, decrease the number of carbon atoms of the alpha hydroxy carboxylic acid by one to obtain the number of carbon atoms of the aldehyde.
structure of lactic acid is as shown below:
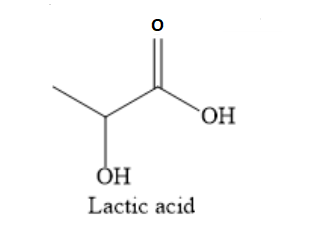
Lactic acid contains 3 carbon atoms. The corresponding aldehyde will have 3-1=2 carbon atoms.
In the given options for aldehydes, the number of carbon atoms in benzaldehyde, formaldehyde, acetaldehyde and propionaldehyde is 7,1, 2, and 3 respectively. Acetaldehyde contains 2 carbon atoms.
Hence, option C) \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\] is the correct answer.
Note:
Since cyano group is added to carbonyl group, cyanohydrin has one excess carbon atom than corresponding aldehyde. Hence, while determining the structure of the starting aldehyde, do not forget to account for additional carbon atoms from the cyano group.
Complete step by step answer:
The aldehydes react with hydrogen cyanide to form cyanohydrin.

In the above reaction, ‘R’ represents an alkyl group. The cyanohydrin has an additional carbon atom than aldehyde. This additional carbon atom comes from hydrogen cyanide. The cyano group of hydrogen cyanide is added to carbonyl carbon and the hydrogen atom of hydrogen cyanide is added to carbonyl oxygen. A new carbon-carbon single bond is formed and the carbon-oxygen double bond is converted into a single bond. Aldehyde contains carbonyl groups whereas cyanohydrin contains one hydroxyl group and one cyano group.
The hydrolysis of cyanohydrin gives alpha hydroxy, carboxylic acids.

In the above reaction, the cyano group is hydrolyzed to a carboxylic acid functional group. The number of carbon atoms in the alpha hydroxy carboxylic acid is the same as the number of carbon atoms in the cyanohydrin.
When an alpha hydroxy carboxylic acid is given and it is asked to determine the structure of aldehyde, whose cyanohydrin upon hydrolysis will give the given alpha hydroxy carboxylic acid, then, decrease the number of carbon atoms of the alpha hydroxy carboxylic acid by one to obtain the number of carbon atoms of the aldehyde.
structure of lactic acid is as shown below:

Lactic acid contains 3 carbon atoms. The corresponding aldehyde will have 3-1=2 carbon atoms.
In the given options for aldehydes, the number of carbon atoms in benzaldehyde, formaldehyde, acetaldehyde and propionaldehyde is 7,1, 2, and 3 respectively. Acetaldehyde contains 2 carbon atoms.
Hence, option C) \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\] is the correct answer.
Note:
Since cyano group is added to carbonyl group, cyanohydrin has one excess carbon atom than corresponding aldehyde. Hence, while determining the structure of the starting aldehyde, do not forget to account for additional carbon atoms from the cyano group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




