
How do ‘d’ orbitals split in an octahedral crystal field?
Answer
583.5k+ views
Hint: All the valence orbitals of the metal cations are of the same energy(all the d orbitals have the same energy). We already know the crystal field theory called CFT. Crystal field theory states that the interaction between metal cations and ligands are electrostatic. The repulsive forces are the reason for the splitting of d orbitals.
Complete step by step answer:
We know that there are 5 lobes in the d orbital. They are ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$, ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$, ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$
-Electrons are filled subsequently in all these lobes.
-All the orbitals have the same energy which means that they are degenerate. But when a ligand is approaching the metal, the degeneracy is lost.
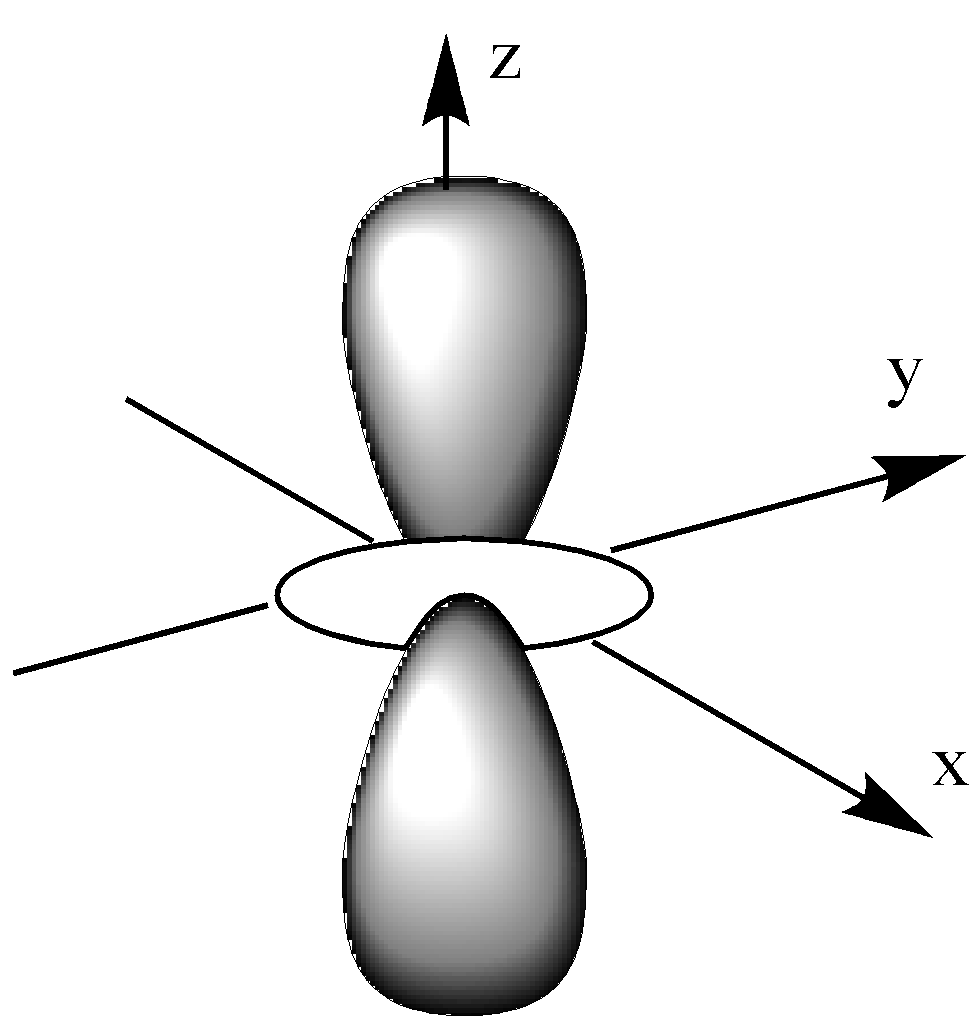
-The given below is ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ orbital. This means that it is in the z-axis and xy plane.
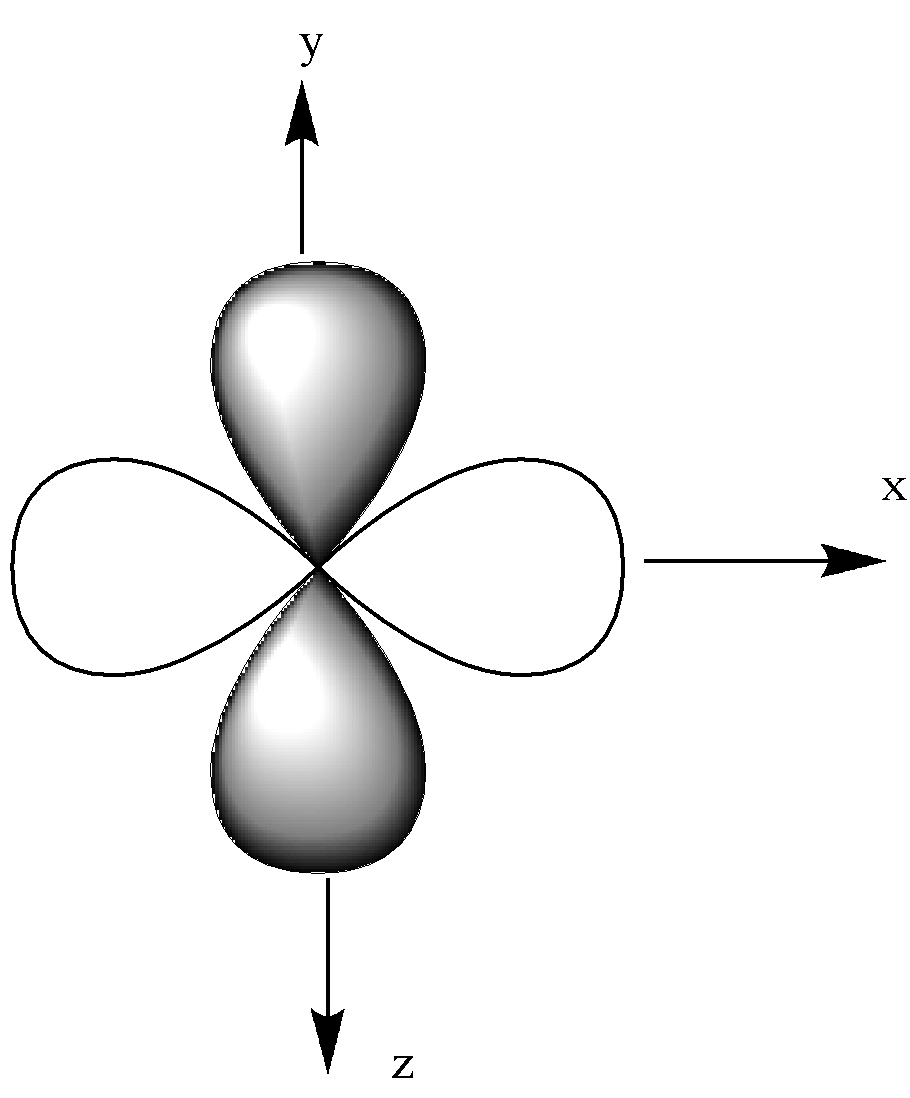
-The given below is ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$ orbital.
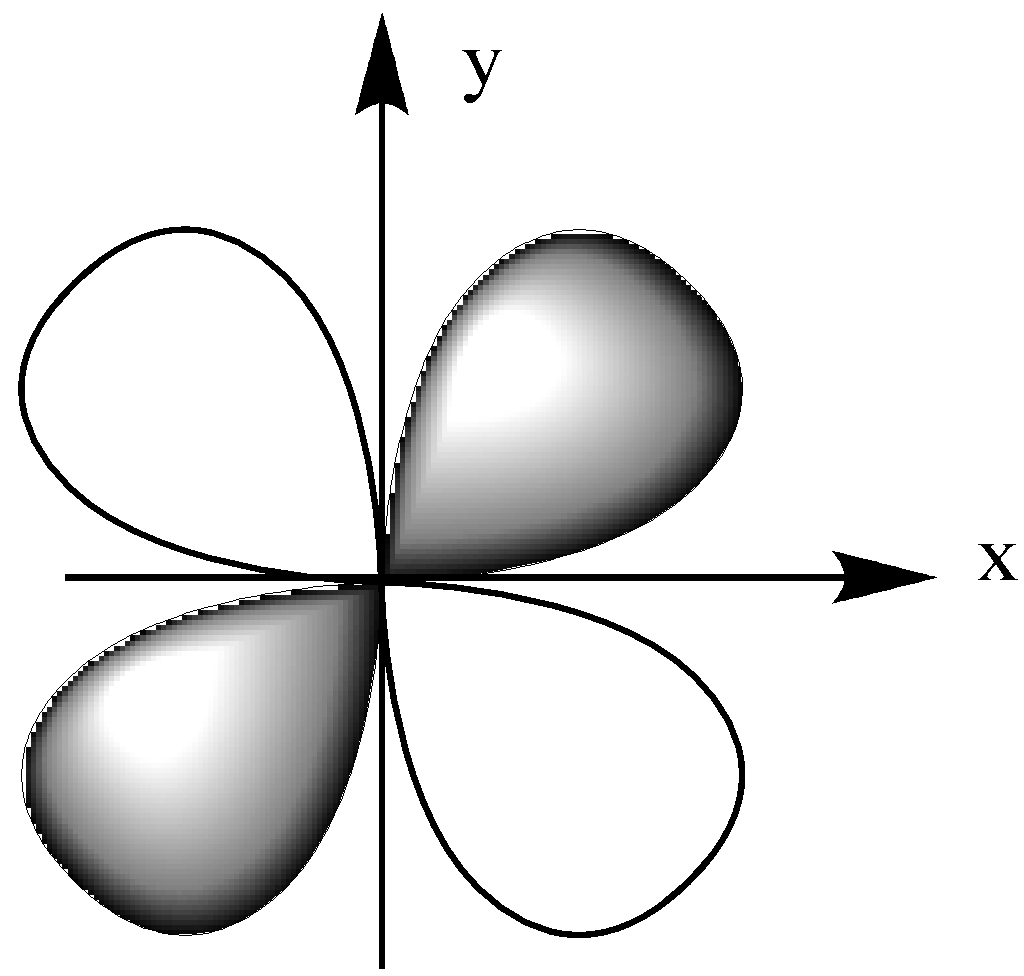
-Now, let us look at ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$.
-We already know that ligands are negatively charged and are coming to the metal cation through the axis.
-When a ligand is coming through the axis, the ligands overlap with ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ orbitals since its lobes are lying on the axis. This will result in strong repulsion between the negative charge of electrons and the ligands in the axis.
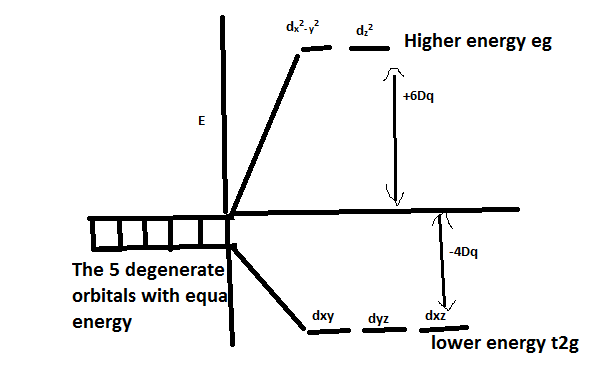
-As we have already learnt in CFT, this repulsive force leads to splitting of the d orbital into higher energy ${{\text{e}}_{\text{g}}}$ and lower energy ${{\text{t}}_{{\text{2g}}}}$ levels.
-When ligands are approaching through the planes, it undergoes less repulsion and thus split into ${{\text{t}}_{{\text{2g}}}}$ levels. This happens when the ligand is approaching through xy, xz and yz planes. The orbital lobes which are in this plane are ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$ .
-So, In an octahedral Crystal field, d orbitals split into higher energy ${{\text{e}}_{\text{g}}}$ and lower energy ${{\text{t}}_{{\text{2g}}}}$
Note:The higher energy orbitals are those which undergo higher repulsion since its orbitals overlap with the approaching ligands. It is the opposite of the tetrahedral complex. In tetrahedral splitting, the higher energy is ${{\text{t}}_{{\text{2g}}}}$ and lower energy is ${{\text{e}}_{\text{g}}}$.
Complete step by step answer:
We know that there are 5 lobes in the d orbital. They are ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$, ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$, ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$
-Electrons are filled subsequently in all these lobes.
-All the orbitals have the same energy which means that they are degenerate. But when a ligand is approaching the metal, the degeneracy is lost.
-The given below is ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ orbital. This means that it is in the z-axis and xy plane.

-The given below is ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$ orbital.

It is in the x and y-axis.
-Now, let us look at ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$.

Similarly, ${{\text{d}}_{{\text{xz}}}}$ lies in the plane xz, ${{\text{d}}_{{\text{yz}}}}$ lies in the plane yz.
-We already know that ligands are negatively charged and are coming to the metal cation through the axis.
-When a ligand is coming through the axis, the ligands overlap with ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ orbitals since its lobes are lying on the axis. This will result in strong repulsion between the negative charge of electrons and the ligands in the axis.
-As we have already learnt in CFT, this repulsive force leads to splitting of the d orbital into higher energy ${{\text{e}}_{\text{g}}}$ and lower energy ${{\text{t}}_{{\text{2g}}}}$ levels.
-When ligands are approaching through the planes, it undergoes less repulsion and thus split into ${{\text{t}}_{{\text{2g}}}}$ levels. This happens when the ligand is approaching through xy, xz and yz planes. The orbital lobes which are in this plane are ${{\text{d}}_{{\text{xy}}}}$, ${{\text{d}}_{{\text{xz}}}}$, ${{\text{d}}_{{\text{yz}}}}$ .
-So, In an octahedral Crystal field, d orbitals split into higher energy ${{\text{e}}_{\text{g}}}$ and lower energy ${{\text{t}}_{{\text{2g}}}}$

Note:The higher energy orbitals are those which undergo higher repulsion since its orbitals overlap with the approaching ligands. It is the opposite of the tetrahedral complex. In tetrahedral splitting, the higher energy is ${{\text{t}}_{{\text{2g}}}}$ and lower energy is ${{\text{e}}_{\text{g}}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




