
Draw the diagram showing micelle formation by the following detergent. \[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\]
Answer
522.9k+ views
Hint: Sodium lauryl sulphate \[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\] is an anionic detergent.
Micelles are lipid molecules that form spherical structures in aqueous solutions. Micelle formation is a reaction of fatty acids' amphipathic composition, which means they have both hydrophilic (polar head groups) and hydrophobic (polar tail groups) areas (the long hydrophobic chain).
Complete answer:
Micelles are lipid molecules that form spherical structures in aqueous solutions. Micelle formation is a reaction of fatty acids' amphipathic composition, which means they have both hydrophilic (polar head groups) and hydrophobic (polar tail groups) areas (the long hydrophobic chain).
Micelles are made up of amphiphilic molecules that self-assemble. Micelles are formed in aqueous solution, with the polar region facing the micelle's outer surface and the nonpolar region becoming the micelle's nucleus. Both hydrophilic and hydrophobic agents can be delivered by micelles.
Sodium lauryl sulphate \[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\] is an anionic detergent.
Sodium lauryl sulphate is a surfactant that is used in a number of cosmetic, dermatological, and consumer goods due to its potency, low cost, abundance, and versatility. SLS is most often present in your toothpaste, shampoo, and body wash.
When mixed with water, it dissociates into the following:
\[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\xrightarrow{{{H_2}O}}C{H_3}{(C{H_2})_{10}}C{H_2}OS{O_3}^ - + N{a^ + }\]
With their \[ - OS{O_3}^ - \] groups in water, these anions are found on the surface, while the hydrocarbon component stays away from it and remains at the surface.
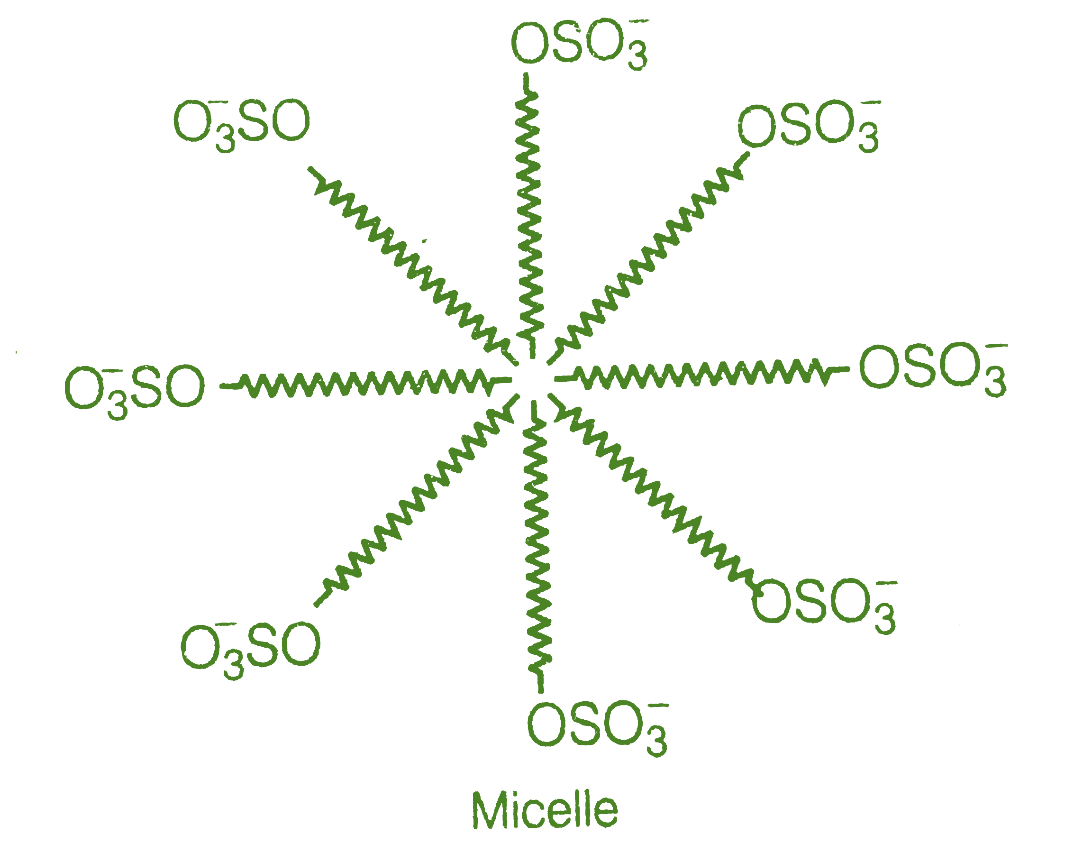
At higher concentrations, these anions are drawn into the bulk of the solution and form a spherical aggregate on the surface of the sphere, with the hydrocarbon component pointed towards the centre and the \[ - OS{O_3}^ - \] part pointing outwards.
Micelle is the name given to the resulting aggregate.
Note:
At higher concentrations, these anions are drawn into the bulk of the solution and form a spherical aggregate on the surface of the sphere, with the hydrocarbon component pointed towards the centre and the \[ - OS{O_3}^ - \] part pointing outwards.
Micelles are lipid molecules that form spherical structures in aqueous solutions. Micelle formation is a reaction of fatty acids' amphipathic composition, which means they have both hydrophilic (polar head groups) and hydrophobic (polar tail groups) areas (the long hydrophobic chain).
Complete answer:
Micelles are lipid molecules that form spherical structures in aqueous solutions. Micelle formation is a reaction of fatty acids' amphipathic composition, which means they have both hydrophilic (polar head groups) and hydrophobic (polar tail groups) areas (the long hydrophobic chain).
Micelles are made up of amphiphilic molecules that self-assemble. Micelles are formed in aqueous solution, with the polar region facing the micelle's outer surface and the nonpolar region becoming the micelle's nucleus. Both hydrophilic and hydrophobic agents can be delivered by micelles.
Sodium lauryl sulphate \[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\] is an anionic detergent.
Sodium lauryl sulphate is a surfactant that is used in a number of cosmetic, dermatological, and consumer goods due to its potency, low cost, abundance, and versatility. SLS is most often present in your toothpaste, shampoo, and body wash.
When mixed with water, it dissociates into the following:
\[C{H_3}{(C{H_2})_{10}}C{H_2}OS{\overline O _3}N{a^ + }\xrightarrow{{{H_2}O}}C{H_3}{(C{H_2})_{10}}C{H_2}OS{O_3}^ - + N{a^ + }\]
With their \[ - OS{O_3}^ - \] groups in water, these anions are found on the surface, while the hydrocarbon component stays away from it and remains at the surface.
At higher concentrations, these anions are drawn into the bulk of the solution and form a spherical aggregate on the surface of the sphere, with the hydrocarbon component pointed towards the centre and the \[ - OS{O_3}^ - \] part pointing outwards.
Micelle is the name given to the resulting aggregate.

Note:
At higher concentrations, these anions are drawn into the bulk of the solution and form a spherical aggregate on the surface of the sphere, with the hydrocarbon component pointed towards the centre and the \[ - OS{O_3}^ - \] part pointing outwards.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




