
Draw the Lewis structure of the following compounds.
(1) \[L{i_2}O\]
(2) \[CaC{l_2}\]
(3) LiF
Answer
565.2k+ views
Hint: Lewis structure shows how the valence electrons are arranged above the atom of the molecule. The given compounds are ionic compounds therefore transfer of electrons takes place to achieve the stable octet.
Complete answer:
The Lewis structure is the representation of valence electrons around the molecule. The Lewis structure shows how the electrons arranged around the atom of the molecule.
(1) \[L{i_2}O\]- In lithium oxide, two lithium atoms and one oxygen atom is present. The atomic number of lithium is 3 and the electronic configuration of lithium is \[[He]2{s^1}\]. The atomic number of oxygen is 8 and the electronic configuration of oxygen is \[[He]2{s^2}2{p^4}\]. The valence electron in lithium is 1 and the valence electrons in oxygen is 6. Each lithium atom loses one electron to form lithium cation \[L{i^ + }\] and total two electrons are lost and oxygen gains two electrons to form oxygen anion \[{O^{2 - }}\].
The Lewis structure of \[L{i_2}O\] is shown below.
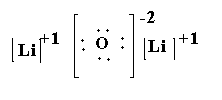
(2) \[CaC{l_2}\]-In calcium chloride, two chlorine atoms and one calcium atoms are present. The atomic number of chlorine is 17 and the electronic configuration of chlorine is \[[Ne]3{s^2}3{p^5}\]. The atomic number of calcium is 20 and the electronic configuration of calcium is \[[Ar]4{s^2}\]. The valence electrons in chlorine are 7 and the valence electrons in calcium are 2. The calcium transfers its two electrons to the chlorine atom and become calcium cation \[C{a^{2 + }}\]and each chlorine becomes anion \[C{l^ - }\].
The Lewis structure of \[CaC{l_2}\] is shown below.
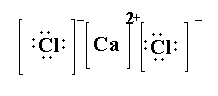
(3) LiF- In lithium fluoride one lithium atom and one fluoride atom is present. The atomic number of lithium is 3 and the electronic configuration of lithium is \[[He]2{s^1}\]. The atomic weight of fluorine is 9 and the electronic configuration of fluorine is \[[He]2{s^2}2{p^5}\]. The valence electrons in lithium is 1 and the valence electrons in fluorine is 7. Here lithium transfers its one electron to fluorine to form \[L{i^ + }\] and fluorine forms \[{F^ - }\].
The Lewis structure of LiF is shown below.
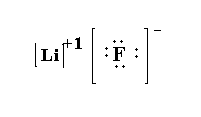
Note:
In covalent compound sharing of electrons takes place where bonding electrons are represented by chemical bonds and non-bonding are represented by lone pairs.
Complete answer:
The Lewis structure is the representation of valence electrons around the molecule. The Lewis structure shows how the electrons arranged around the atom of the molecule.
(1) \[L{i_2}O\]- In lithium oxide, two lithium atoms and one oxygen atom is present. The atomic number of lithium is 3 and the electronic configuration of lithium is \[[He]2{s^1}\]. The atomic number of oxygen is 8 and the electronic configuration of oxygen is \[[He]2{s^2}2{p^4}\]. The valence electron in lithium is 1 and the valence electrons in oxygen is 6. Each lithium atom loses one electron to form lithium cation \[L{i^ + }\] and total two electrons are lost and oxygen gains two electrons to form oxygen anion \[{O^{2 - }}\].
The Lewis structure of \[L{i_2}O\] is shown below.
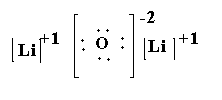
(2) \[CaC{l_2}\]-In calcium chloride, two chlorine atoms and one calcium atoms are present. The atomic number of chlorine is 17 and the electronic configuration of chlorine is \[[Ne]3{s^2}3{p^5}\]. The atomic number of calcium is 20 and the electronic configuration of calcium is \[[Ar]4{s^2}\]. The valence electrons in chlorine are 7 and the valence electrons in calcium are 2. The calcium transfers its two electrons to the chlorine atom and become calcium cation \[C{a^{2 + }}\]and each chlorine becomes anion \[C{l^ - }\].
The Lewis structure of \[CaC{l_2}\] is shown below.
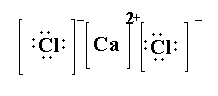
(3) LiF- In lithium fluoride one lithium atom and one fluoride atom is present. The atomic number of lithium is 3 and the electronic configuration of lithium is \[[He]2{s^1}\]. The atomic weight of fluorine is 9 and the electronic configuration of fluorine is \[[He]2{s^2}2{p^5}\]. The valence electrons in lithium is 1 and the valence electrons in fluorine is 7. Here lithium transfers its one electron to fluorine to form \[L{i^ + }\] and fluorine forms \[{F^ - }\].
The Lewis structure of LiF is shown below.
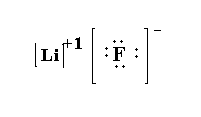
Note:
In covalent compound sharing of electrons takes place where bonding electrons are represented by chemical bonds and non-bonding are represented by lone pairs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




