
Draw the structure of the monomer for each of the following polymers:
1. Nylon 6
2. Polypropene
Answer
597.3k+ views
Hint: We know that these two are polymers. We have to draw the structures of the single unit, so that by repeating those units we can get these polymers.
Step by step solution:
We know that a monomer is a molecule that can be bonded to other identical molecules to form a polymer.
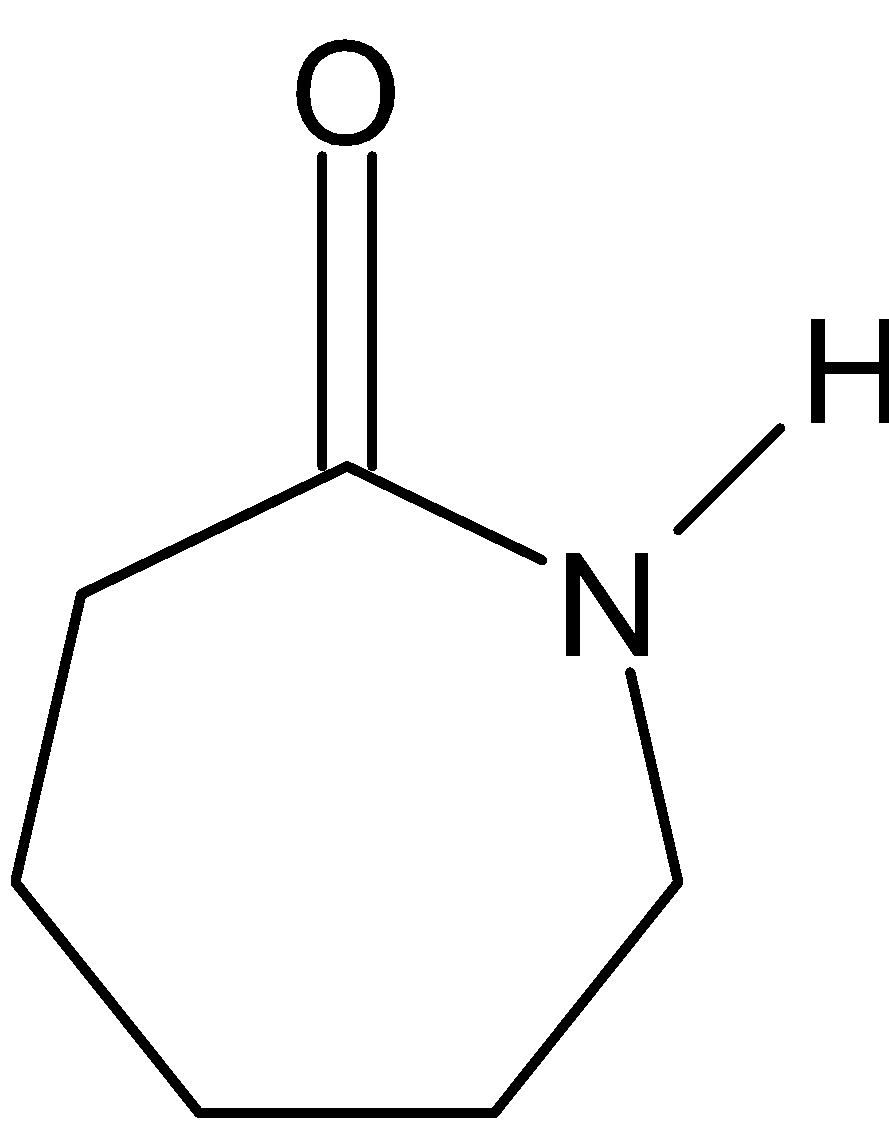
(1) Nylon 6: It is also known as polycaprolactam. It is a polymer formed by ring – opening polymerization of caprolactam. So, the polymer of Nylon 6 is g-caprolactam. During polymerization, the amide bond within each caprolactam molecule is broken. Here is the structure:
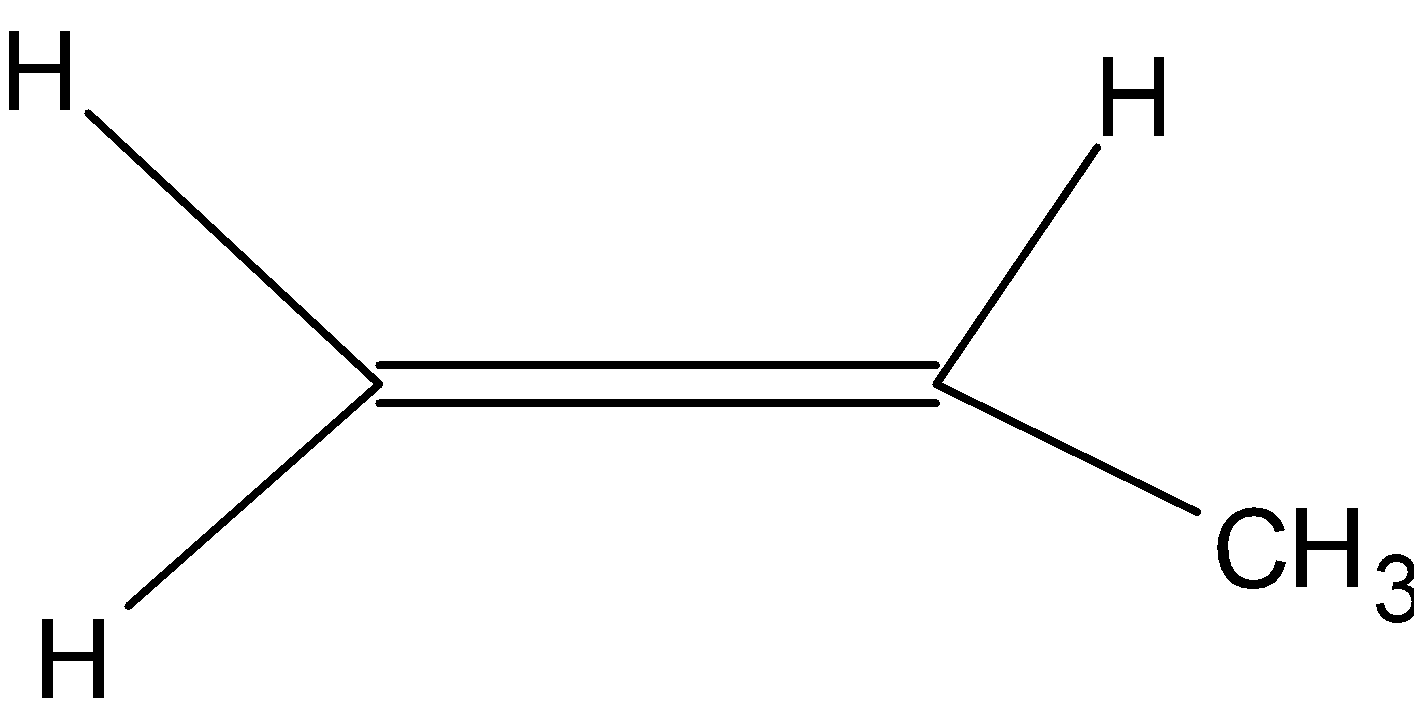
(2) Polypropene: It is also known as polypropene. It is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene. It is made by addition polymerization. It is used in a variety of applications to include packaging for consumer products, plastic parts for various industries including the automotive industry, special devices like living hinges, and textiles. Here is the structure of monomer:
Note: Nylon 6 is not a condensation polymer. Polypropylene belongs to the group of polyolefins and is partially crystalline and non-polar. Polypropylene (PP) is a thermoplastic.
Step by step solution:
We know that a monomer is a molecule that can be bonded to other identical molecules to form a polymer.
(1) Nylon 6: It is also known as polycaprolactam. It is a polymer formed by ring – opening polymerization of caprolactam. So, the polymer of Nylon 6 is g-caprolactam. During polymerization, the amide bond within each caprolactam molecule is broken. Here is the structure:

(2) Polypropene: It is also known as polypropene. It is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene. It is made by addition polymerization. It is used in a variety of applications to include packaging for consumer products, plastic parts for various industries including the automotive industry, special devices like living hinges, and textiles. Here is the structure of monomer:

Note: Nylon 6 is not a condensation polymer. Polypropylene belongs to the group of polyolefins and is partially crystalline and non-polar. Polypropylene (PP) is a thermoplastic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




