
Draw the structures of fructose for Haworth projection formulae.
Answer
588k+ views
Hint: The sugars can be written using Haworth's formula. Pyranose and Furanose are the oxides rings that resemble the oxygen-containing heterocyclic ring pyran and furan respectively. These are five or six-membered rings where one of the positions is occupied by oxide. The Furanose structure is similar to the 5-membered heterocyclic ring furan.
Complete step by step solution:
Fructose occurs in fruits and it is called the fruit sugar. It is also present in honey and sweet fruits along with glucose. In the combined state, it is also present in disaccharide and polysaccharide (insulin).
Its molecular formula is $\text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}$. It contains the keto group at C-2 and the six carbon atoms are arranged in a straight chain. The Fischer projection of the fructose can be converted into the cyclic structure.
Like glucose, fructose has a cyclic structure. The hemiacetal is formed by the intramolecular combination of ${{\text{C}}_{\text{2}}}$ , keto group and $-\text{OH}$ a group of ${{\text{C}}_{6}}$ the atom. As a result, ${{\text{C}}_{\text{2}}}$ the atom becomes asymmetric and therefore D-fructose has two possible isomers as a $\text{ }\!\!\alpha\!\!\text{ -D-fructose}$ and $\text{ }\!\!\beta\!\!\text{ -D-fructose}$.
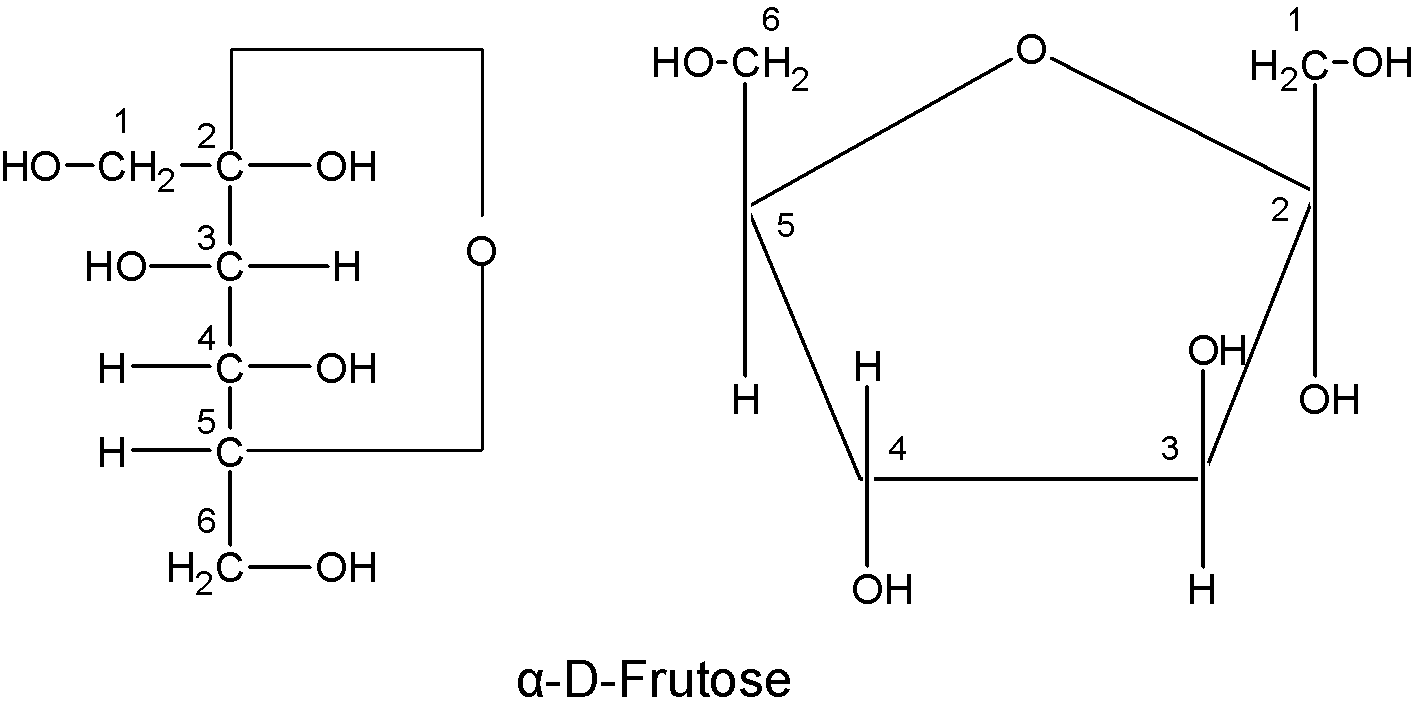
These structures were suggested by Haworth and are known as the Haworth projection formulae or pyranose structures.
The $\text{ }\!\!\beta\!\!\text{ -D-fructose}$ structure is as follows:
To write the pyranose structures for any monosaccharide first, draw a pentagon with its oxygen atom at the top. The terminal $\text{-C}{{\text{H}}_{\text{2}}}\text{OH}$ group as shown in the figure is always placed above the plane of the pentagon ring. Place all the groups which are present on the left-hand side in Fischer projection above the plane of the ring and all those groups on the right hand in Fischer projection below the plane of the ring.
Pyranose rings are formed by the reaction of the keto group on the carbon number two of sugar with the hydroxyl which is at the carbon one. This formation goes through the hemiacetal. This structure is similar to the furan ring. The hemiacetal structure is as shown below:
Thus here we know that the five-membered rings of oxide are called the pyranose. Due to the formation of an oxide ring, the new asymmetric carbon atom is created at the carbonyl carbon, which is called an anomeric carbon atom.
Two different configurations are possible at the anomeric carbon atom and they are called the anomers.
Here, the two structures shown above are all similar except at the C-2 carbon atom. In $\text{ }\!\!\alpha\!\!\text{ -D-fructose}$ the hydroxyl ion below the plane and $\text{ }\!\!\beta\!\!\text{ -D-fructose}$ the hydroxyl ion is above the plane.
Note: Remember that groups which are on the right in a Fischer projection are written down in Haworth projection and groups which are on the left in a Fischer projection are up in a Haworth projection. The fructose belongs to the D-series and is a laevorotatory compound. It is also called laevulose.
Complete step by step solution:
Fructose occurs in fruits and it is called the fruit sugar. It is also present in honey and sweet fruits along with glucose. In the combined state, it is also present in disaccharide and polysaccharide (insulin).
Its molecular formula is $\text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}$. It contains the keto group at C-2 and the six carbon atoms are arranged in a straight chain. The Fischer projection of the fructose can be converted into the cyclic structure.
Like glucose, fructose has a cyclic structure. The hemiacetal is formed by the intramolecular combination of ${{\text{C}}_{\text{2}}}$ , keto group and $-\text{OH}$ a group of ${{\text{C}}_{6}}$ the atom. As a result, ${{\text{C}}_{\text{2}}}$ the atom becomes asymmetric and therefore D-fructose has two possible isomers as a $\text{ }\!\!\alpha\!\!\text{ -D-fructose}$ and $\text{ }\!\!\beta\!\!\text{ -D-fructose}$.

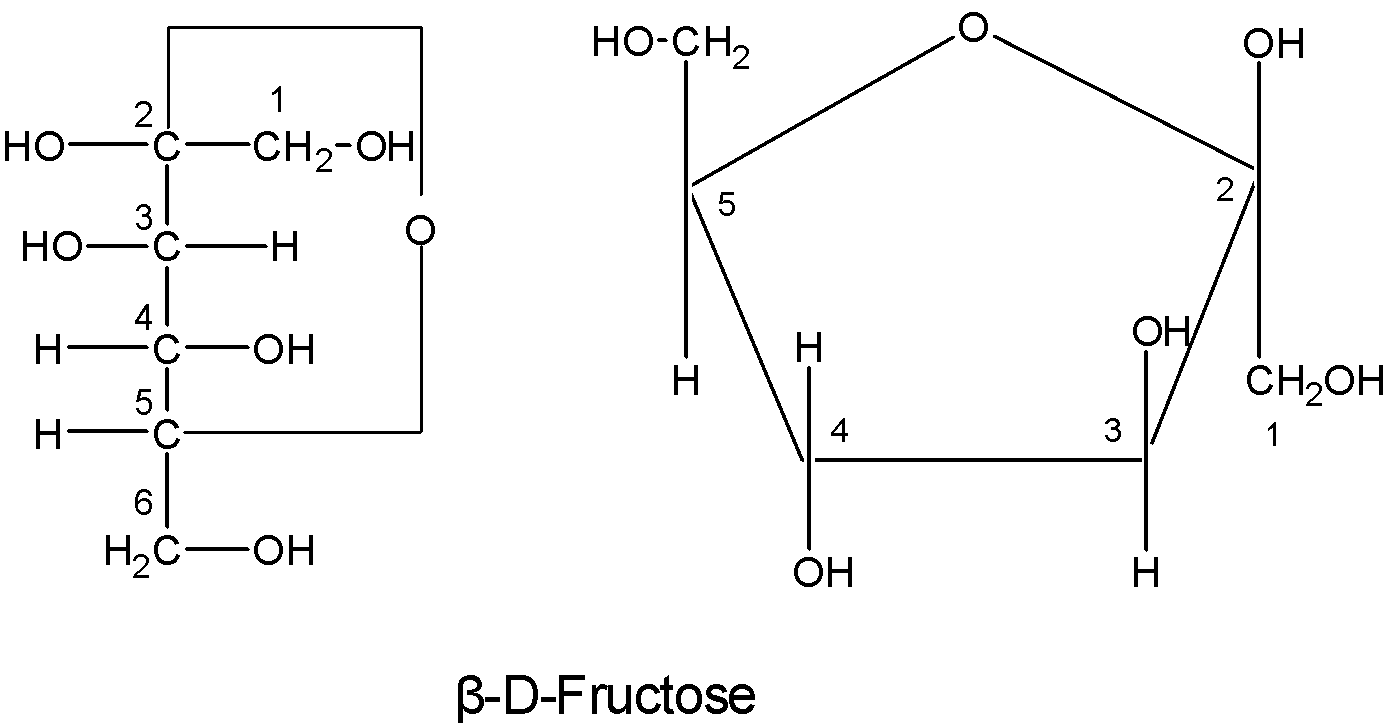
These structures were suggested by Haworth and are known as the Haworth projection formulae or pyranose structures.
The $\text{ }\!\!\beta\!\!\text{ -D-fructose}$ structure is as follows:

To write the pyranose structures for any monosaccharide first, draw a pentagon with its oxygen atom at the top. The terminal $\text{-C}{{\text{H}}_{\text{2}}}\text{OH}$ group as shown in the figure is always placed above the plane of the pentagon ring. Place all the groups which are present on the left-hand side in Fischer projection above the plane of the ring and all those groups on the right hand in Fischer projection below the plane of the ring.
Pyranose rings are formed by the reaction of the keto group on the carbon number two of sugar with the hydroxyl which is at the carbon one. This formation goes through the hemiacetal. This structure is similar to the furan ring. The hemiacetal structure is as shown below:
Thus here we know that the five-membered rings of oxide are called the pyranose. Due to the formation of an oxide ring, the new asymmetric carbon atom is created at the carbonyl carbon, which is called an anomeric carbon atom.
Two different configurations are possible at the anomeric carbon atom and they are called the anomers.
Here, the two structures shown above are all similar except at the C-2 carbon atom. In $\text{ }\!\!\alpha\!\!\text{ -D-fructose}$ the hydroxyl ion below the plane and $\text{ }\!\!\beta\!\!\text{ -D-fructose}$ the hydroxyl ion is above the plane.
Note: Remember that groups which are on the right in a Fischer projection are written down in Haworth projection and groups which are on the left in a Fischer projection are up in a Haworth projection. The fructose belongs to the D-series and is a laevorotatory compound. It is also called laevulose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




