
Ethyl Acetate reacts with $C{H_3}MgBr$ ?
A.Secondary alcohols
B.Tertiary alcohols
C.Primary alcohols and acids
D.An acid
Answer
569.1k+ views
Hint: Ethyl acetate is an ester whose molecular formula is $C{H_3}COO{C_2}{H_5}$. The other reagent mentioned in the question is methyl magnesium bromide. It is a Grignard reagent.
Complete step by step answer:
The reaction of Ethyl acetate reacts with $C{H_3}MgBr$ involves the reaction of an ester with an organometallic compound that is known as methyl magnesium bromide. This compound will react with ethyl acetate to form an intermediate compound as shown in the diagram below:
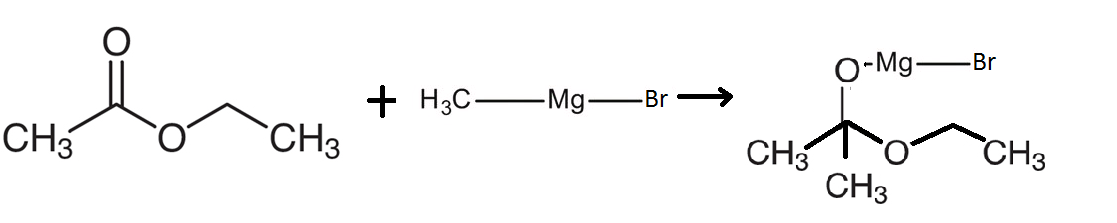
This reaction shows that methyl magnesium bromide will split into methyl groups on one side and the magnesium bromide on the other side. The magnesium bromide reacts with the oxygen which is bonded to the carbon using a double bond. The other methyl group bonds with the carbon on the other side. Therefore, the following compound is formed as shown in the figure.
This compound is an intermediate and hence short lived. This intermediate will now separate to form the following products as shown in the diagram below:
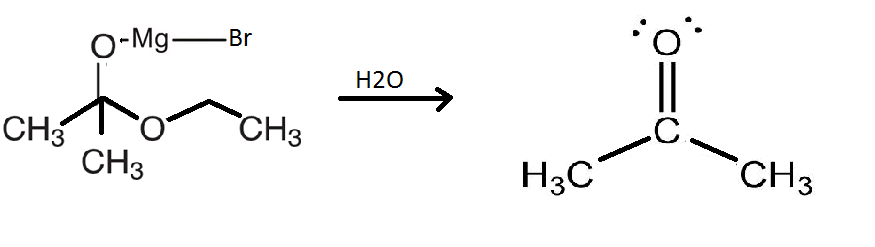
Therefore, the intermediate will release a water molecule to form propanone which is a ketone.
Now this ketone will combine again with a methyl magnesium bromide to form an alcohol. This is demonstrated below:
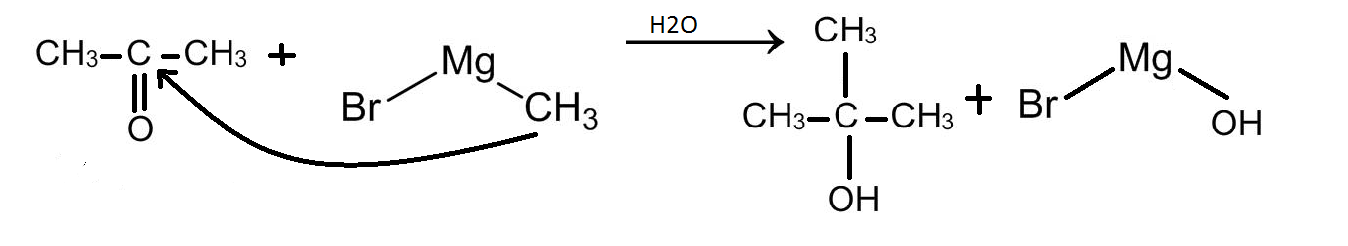
Therefore, the alkyl part of the second methyl magnesium bromide molecule will attack propanone at the carbonyl carbon that is the middle carbon as shown in the reaction above. Therefore, the methyl group will attach itself to the middle carbon. The oxygen will receive a negative charge and this negative charge of the oxygen will be satisfied by a hydrogen ion from a water molecule.
Therefore, this reaction shows that a secondary alcohol is formed. This alcohol is known as $2$ - methyl propan- $2$ - ol. Therefore, the answer to this question will be option a that is, a secondary alcohol is formed.
Note: The reaction of an ester with a Grignard reaction leads to the formation of a ketone.
-If this ketone is exposed to an excess of the same Grignard reagent it will form a secondary alcohol.
-The Grignard reagent separates into the alkyl part and the magnesium bromide part just before reacting.
Complete step by step answer:
The reaction of Ethyl acetate reacts with $C{H_3}MgBr$ involves the reaction of an ester with an organometallic compound that is known as methyl magnesium bromide. This compound will react with ethyl acetate to form an intermediate compound as shown in the diagram below:
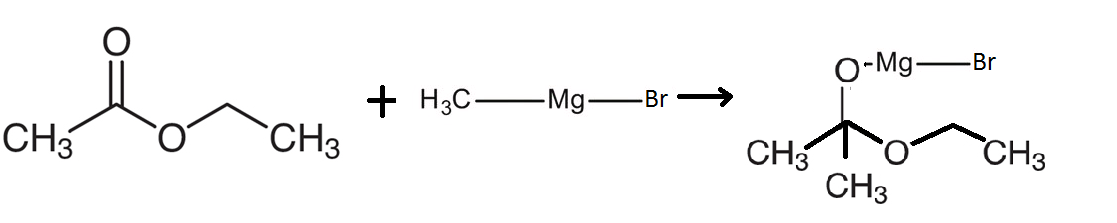
This reaction shows that methyl magnesium bromide will split into methyl groups on one side and the magnesium bromide on the other side. The magnesium bromide reacts with the oxygen which is bonded to the carbon using a double bond. The other methyl group bonds with the carbon on the other side. Therefore, the following compound is formed as shown in the figure.
This compound is an intermediate and hence short lived. This intermediate will now separate to form the following products as shown in the diagram below:

Therefore, the intermediate will release a water molecule to form propanone which is a ketone.
Now this ketone will combine again with a methyl magnesium bromide to form an alcohol. This is demonstrated below:

Therefore, the alkyl part of the second methyl magnesium bromide molecule will attack propanone at the carbonyl carbon that is the middle carbon as shown in the reaction above. Therefore, the methyl group will attach itself to the middle carbon. The oxygen will receive a negative charge and this negative charge of the oxygen will be satisfied by a hydrogen ion from a water molecule.
Therefore, this reaction shows that a secondary alcohol is formed. This alcohol is known as $2$ - methyl propan- $2$ - ol. Therefore, the answer to this question will be option a that is, a secondary alcohol is formed.
Note: The reaction of an ester with a Grignard reaction leads to the formation of a ketone.
-If this ketone is exposed to an excess of the same Grignard reagent it will form a secondary alcohol.
-The Grignard reagent separates into the alkyl part and the magnesium bromide part just before reacting.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




