
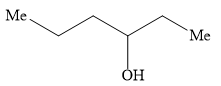
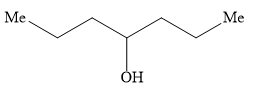
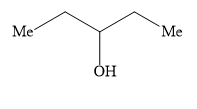
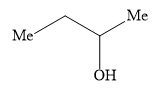
Ethyl formate ester reacts with \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}}\] to give secondary alcohol. The alcohol formed is
A)
B)
C)
D)




Answer
582.6k+ views
Hint: Formic acid contains one carbon atom. Propyl magnesium bromide contains three carbon atoms. The product will have seven carbon atoms.
Complete answer:
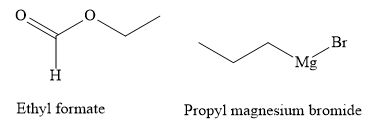
The structures of the starting materials ethyl formate and propyl magnesium bromide are as shown below:
Write the chemical reaction
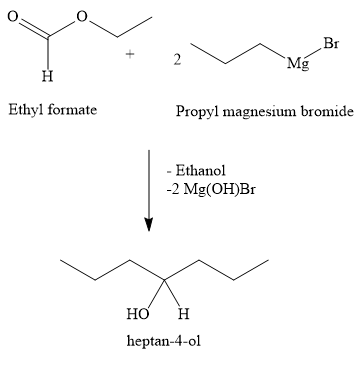
Two moles of propyl magnesium bromide react with one mole of ethyl formate to form heptan-4-ol. Propyl magnesium bromide is a Grignard reagent. Ethyl formate is an ester. Heptan-2-ol is a secondary alcohol. Thus, one mole of ester reacts with two moles of grignard reagent to form one mole of heptan-2-ol. In the reaction, two moles of \[{\text{Mg}}\left( {{\text{OH}}} \right){\text{Br}}\] and one mole of ethanol are the side product.
Hence, the option B ) heptan-4-ol is the correct option.
Additional Information: An ester of formic acid reacts with two equivalents of a grignard reagent to form a secondary alcohol. An ester reacts with two equivalents of a grignard reagent to form a tertiary alcohol. An aldehyde reacts with one equivalent of grignard reagent to form secondary alcohol. A ketone reacts with one equivalent of grignard reagent to form tertiary alcohol.
Note: If only one equivalent of propyl magnesium bromide reacts, then ethyl formate will give butane aldehyde. Since in the question, it is mentioned that the product is a secondary alcohol, two equivalents of propyl magnesium bromide will react with ethyl formate to form heptan-4-ol. In the secondary alcohol, the carbon atom bearing hydroxyl group has one hydrogen atom and two other carbon atoms.
Complete answer:
The structures of the starting materials ethyl formate and propyl magnesium bromide are as shown below:

Write the chemical reaction

Two moles of propyl magnesium bromide react with one mole of ethyl formate to form heptan-4-ol. Propyl magnesium bromide is a Grignard reagent. Ethyl formate is an ester. Heptan-2-ol is a secondary alcohol. Thus, one mole of ester reacts with two moles of grignard reagent to form one mole of heptan-2-ol. In the reaction, two moles of \[{\text{Mg}}\left( {{\text{OH}}} \right){\text{Br}}\] and one mole of ethanol are the side product.
Hence, the option B ) heptan-4-ol is the correct option.
Additional Information: An ester of formic acid reacts with two equivalents of a grignard reagent to form a secondary alcohol. An ester reacts with two equivalents of a grignard reagent to form a tertiary alcohol. An aldehyde reacts with one equivalent of grignard reagent to form secondary alcohol. A ketone reacts with one equivalent of grignard reagent to form tertiary alcohol.
Note: If only one equivalent of propyl magnesium bromide reacts, then ethyl formate will give butane aldehyde. Since in the question, it is mentioned that the product is a secondary alcohol, two equivalents of propyl magnesium bromide will react with ethyl formate to form heptan-4-ol. In the secondary alcohol, the carbon atom bearing hydroxyl group has one hydrogen atom and two other carbon atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




