
Ethylamine dissolves in water due to intermolecular H-bonding.
If this is true enter 1, if false enter 0.
Answer
579k+ views
Hint: Nitrogen is a high electronegative element. Ethylamine contains nitrogen which can make strong intermolecular bonding with hydrogen of another molecule.
Complete step by step answer:
Due to the presence of hydrogen bond between nitrogen and oxygen in ethylamine as shown in the figure below it dissolves in water and hence the statement is true.
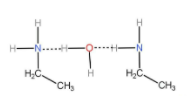
Hydrogen bonding occurs when hydrogen is attached with a highly electronegative atom. The formation of hydrogen bonds is the special case of dipole dipole interaction. Hydrogen bonding is a strong bonding that is shown by the elements nitrogen, fluorine and oxygen due to their high electronegativity value.
There are two types of hydrogen bonding: intermolecular hydrogen bonding and intramolecular hydrogen bonding. In Intermolecular hydrogen bonding the more electronegative atom of 1 molecule forms bond with hydrogen attached with more electronegative element of another molecule whereas in intramolecular hydrogen bonding both more electronegative element and hydrogen are present in the same molecule. The bond is formed within a molecule.
The conditions for hydrogen bonding are that:
1. Molecule must be very electronegative and the hydrogen must link to the high electronegative atom. The more electronegativity, the stronger will be the hydrogen bond.
2. The size of element should be small, the smaller the sizes the greater will be the forces of attraction
Note:
The compounds with hydrogen bonding show abnormally high melting and boiling point. The effect of hydrogen bonding also causes association and association with the molecule. For example carboxylic acid forms dimer due to the presence of hydrogen bonding and HF dissociates due to the hydrogen bonding in HF.
Complete step by step answer:
Due to the presence of hydrogen bond between nitrogen and oxygen in ethylamine as shown in the figure below it dissolves in water and hence the statement is true.

Hydrogen bonding occurs when hydrogen is attached with a highly electronegative atom. The formation of hydrogen bonds is the special case of dipole dipole interaction. Hydrogen bonding is a strong bonding that is shown by the elements nitrogen, fluorine and oxygen due to their high electronegativity value.
There are two types of hydrogen bonding: intermolecular hydrogen bonding and intramolecular hydrogen bonding. In Intermolecular hydrogen bonding the more electronegative atom of 1 molecule forms bond with hydrogen attached with more electronegative element of another molecule whereas in intramolecular hydrogen bonding both more electronegative element and hydrogen are present in the same molecule. The bond is formed within a molecule.
The conditions for hydrogen bonding are that:
1. Molecule must be very electronegative and the hydrogen must link to the high electronegative atom. The more electronegativity, the stronger will be the hydrogen bond.
2. The size of element should be small, the smaller the sizes the greater will be the forces of attraction
Note:
The compounds with hydrogen bonding show abnormally high melting and boiling point. The effect of hydrogen bonding also causes association and association with the molecule. For example carboxylic acid forms dimer due to the presence of hydrogen bonding and HF dissociates due to the hydrogen bonding in HF.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




