
Explain why phenol is more acidic than ethanol?
Answer
577.8k+ views
Hint: Phenol loses its hydrogen ion to form the phenoxide ion which resonates and stabilizes itself and this loss of electrons makes the phenol more acidic than alcohol for example ethanol.
Complete step by step answer:
Acidity of any compound generally depends on its tendency to release hydrogen ions.
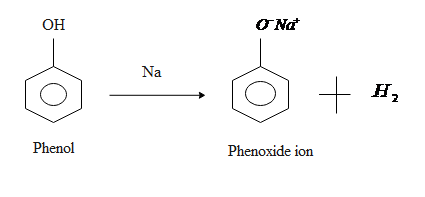
So in case of ethanol it is very tough to remove hydrogen ion from it so we can say that ethanol is less acidic than phenol while on the other side phenol is more acidic than ethanol because it can lose the hydrogen ion very easily because delocalization of electrons takes place in phenols by resonance.
When phenol loses its hydrogen ion it gives phenoxide which is stabilized to some extent as negative charge on the oxygen atom is delocalized around the ring means this negative charge is equally distributed through the compounds or we can say that it is shared by a number of carbon atoms in the benzene ring, therefore the more stable the resulted ion is the more likely is to form.
One more thing is that the presence of the electron withdrawing group increases the acidity of phenol by stabilizing the resulting phenoxide ion and on the other side presence of the electron releasing group decreases the acidity of phenol by destabilizing the phenoxide ion.
Note: Electron withdrawing group draws electron away from the reaction centre while electron releasing group also known as electron donating group releases the electron in the reaction centre.

Complete step by step answer:
Acidity of any compound generally depends on its tendency to release hydrogen ions.
So in case of ethanol it is very tough to remove hydrogen ion from it so we can say that ethanol is less acidic than phenol while on the other side phenol is more acidic than ethanol because it can lose the hydrogen ion very easily because delocalization of electrons takes place in phenols by resonance.
When phenol loses its hydrogen ion it gives phenoxide which is stabilized to some extent as negative charge on the oxygen atom is delocalized around the ring means this negative charge is equally distributed through the compounds or we can say that it is shared by a number of carbon atoms in the benzene ring, therefore the more stable the resulted ion is the more likely is to form.
One more thing is that the presence of the electron withdrawing group increases the acidity of phenol by stabilizing the resulting phenoxide ion and on the other side presence of the electron releasing group decreases the acidity of phenol by destabilizing the phenoxide ion.
Note: Electron withdrawing group draws electron away from the reaction centre while electron releasing group also known as electron donating group releases the electron in the reaction centre.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




