
Why is furan an aromatic compound?
Answer
521.4k+ views
Hint: The compounds which have a hetero atom in their ring structure of the organic compounds are called heterocyclic compounds. At the same time all hetero compounds are not aromatic in nature. The lone pair of electrons on the heteroatom should be involved to get the aromatic character.
Complete answer:
- In the question it is asked why furan is an aromatic compound.
- First, we should know the structure of furan to get a clear idea about the aromatic character.
- The structure of furan is as follows.
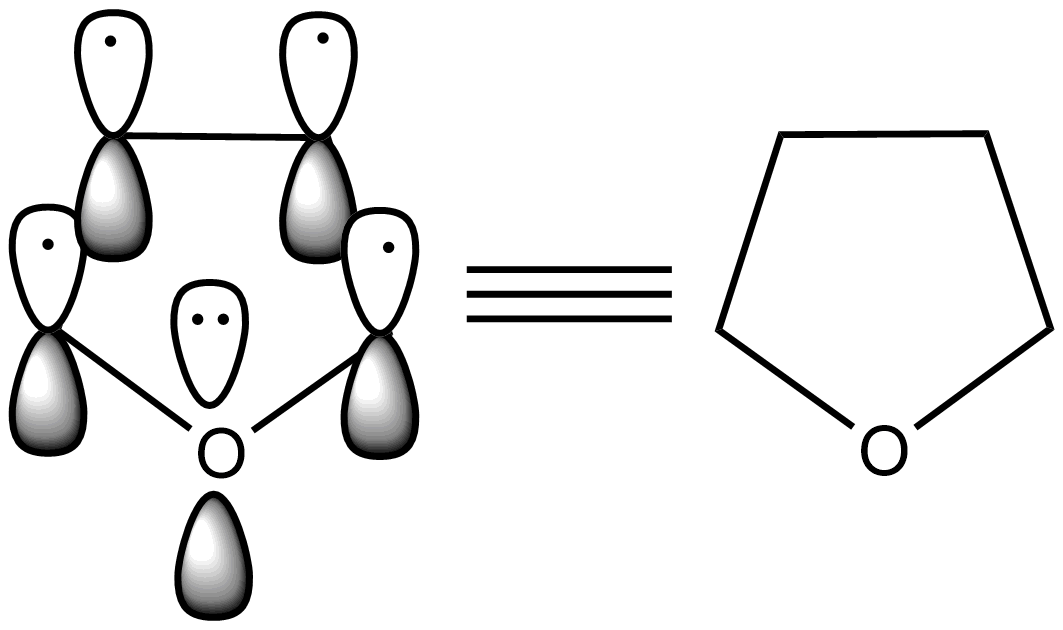
- Now we know that there are two lone pairs of electrons on the oxygen atom which is present in the furan ring.
- Due to the participation of the lone pair of electrons which are in $s{{p}^{2}}$ hybridized orbitals of the oxygen atom with the remaining four electrons which are present in the two bonds of the furan, then it is going to obey the 4n+2 rule to going to act as an aromatic compound.
- Therefore, the lone pair of electrons on the oxygen are reasonable for the aromatic character of the furan.
Note:
All the heterocyclic compounds are not going to exhibit the aromatic character. Only few hetero atomic compounds in which the hetero atom is going to have a lone pair of electrons mostly are going to exhibit the property of the aromatic character. Thiophene is the compound that does not exhibit an aromatic character due to the absence of the lone pair of electrons on the Sulphur atom.
Complete answer:
- In the question it is asked why furan is an aromatic compound.
- First, we should know the structure of furan to get a clear idea about the aromatic character.
- The structure of furan is as follows.
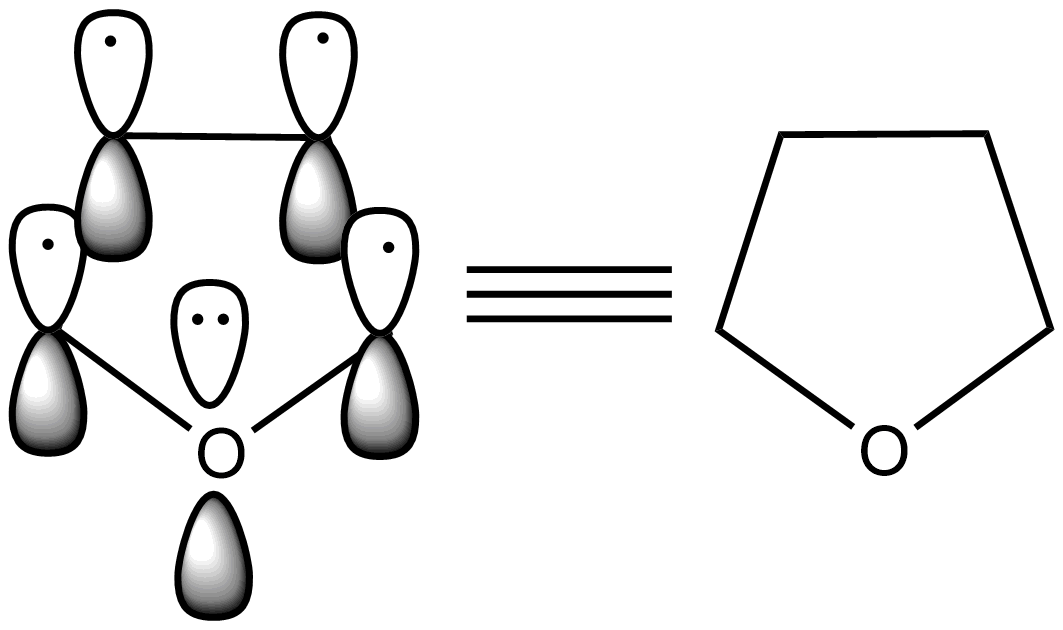
- Now we know that there are two lone pairs of electrons on the oxygen atom which is present in the furan ring.
- Due to the participation of the lone pair of electrons which are in $s{{p}^{2}}$ hybridized orbitals of the oxygen atom with the remaining four electrons which are present in the two bonds of the furan, then it is going to obey the 4n+2 rule to going to act as an aromatic compound.
- Therefore, the lone pair of electrons on the oxygen are reasonable for the aromatic character of the furan.
Note:
All the heterocyclic compounds are not going to exhibit the aromatic character. Only few hetero atomic compounds in which the hetero atom is going to have a lone pair of electrons mostly are going to exhibit the property of the aromatic character. Thiophene is the compound that does not exhibit an aromatic character due to the absence of the lone pair of electrons on the Sulphur atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




