
Gatterrmann reaction is given by:
A. \[{{C}_{6}}{{H}_{6}}\]
B. \[{{C}_{6}}{{H}_{6}}-C{{H}_{3}}\]
C. \[{{C}_{6}}{{H}_{6}}-Cl\]
D. All of these
Answer
608.7k+ views
Hint: We should know that Gattermann reaction is also known by the name of Gattermann formylation. There is a similarity between Gattermann and Friedel-Crafts reaction. It is given by the German chemist, Ludwig Gattermann.
Step by step answer:
We should know about gatterman reaction as a method of formylation of aromatic ring compounds. The gattermann reaction is similar to the Friedel-Crafts reaction.
We use Gattermann reaction for obtaining chlorobenzene or bromobenzene from benzenediazonium chloride by treating it with Cu/HCl or Cu/HBr respectively.
So we will now discuss diazonium salts. The diazonium salts in which, di refers to ‘two’, azo is indicative of ‘nitrogen’ and ium implies that it is cationic in nature. Or we can defined it as the class of organic compounds with general formula R− \[N_{_{2}}^{+}{{X}^{-}}\]where X is an organic or inorganic anion (for example\[,\text{ }C{{l}^{-}},\text{ }B{{r}^{-}},\text{ }BF_{4}^{-},\text{ }etc.\]) and R is an alkyl or aryl group.
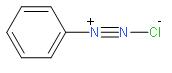
Hence from the structure we observe we can say that they have two nitrogen atoms with one being charged. Benzenediazonium chloride (\[{{C}_{6}}{{H}_{6}}-N_{_{2}}^{+}C{{l}^{-}}\]) benzene diazonium hydrogen sulphate( \[{{C}_{6}}{{H}_{6}}-N_{_{2}}^{+}HSO_{4}^{-}\]) etc. are some of the examples of the diazonium salt that we should remember properly.
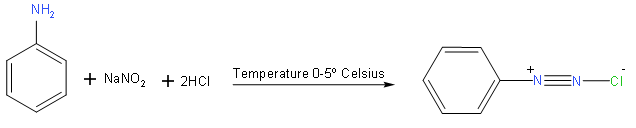
We here use aniline \[{{C}_{6}}{{H}_{6}}-N{{H}_{2}}\] and sodium nitrite with hydrochloric for conversion into benzenediazonium salt,
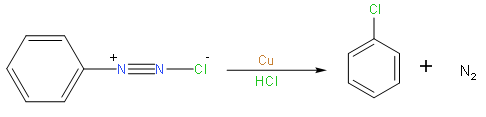
So from the above reaction we can say that we use the Gatterman reaction for the conversion of benzenediazonium to chlorobenzene or bromobenzene.
So our correct option is option C.
Note: We should also know about other examples of Gatterman reaction.
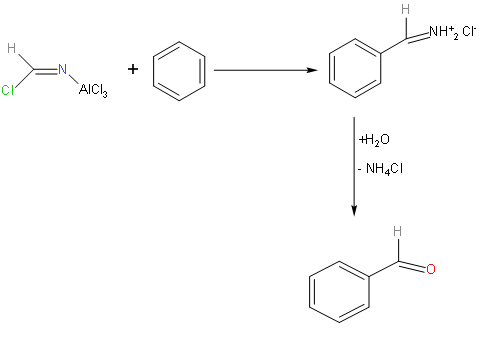
To introduce an aldehyde group to the benzene ring, Gattermann reaction is important in introducing this.
Now we will know about the difference between Sandmeyer reaction and Gatterman reaction. We should know that the Sandmeyer reaction is a type of organic substitution reaction in which we can synthesize aryl halides from aryl diazonium salts while Gattermann reaction is an organic substitution reaction in which we can formulate aromatic compounds. So, the key difference between Sandmeyer reaction and Gattermann reaction is that the Sandmeyer reaction refers to the synthesis of aryl halides from aryl diazonium salts in the presence of copper salts as a catalyst, whereas Gattermann reaction refers to the formylation of aromatic compounds in the presence of a Lewis acid catalyst.
Step by step answer:
We should know about gatterman reaction as a method of formylation of aromatic ring compounds. The gattermann reaction is similar to the Friedel-Crafts reaction.
We use Gattermann reaction for obtaining chlorobenzene or bromobenzene from benzenediazonium chloride by treating it with Cu/HCl or Cu/HBr respectively.
So we will now discuss diazonium salts. The diazonium salts in which, di refers to ‘two’, azo is indicative of ‘nitrogen’ and ium implies that it is cationic in nature. Or we can defined it as the class of organic compounds with general formula R− \[N_{_{2}}^{+}{{X}^{-}}\]where X is an organic or inorganic anion (for example\[,\text{ }C{{l}^{-}},\text{ }B{{r}^{-}},\text{ }BF_{4}^{-},\text{ }etc.\]) and R is an alkyl or aryl group.
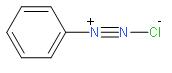
Hence from the structure we observe we can say that they have two nitrogen atoms with one being charged. Benzenediazonium chloride (\[{{C}_{6}}{{H}_{6}}-N_{_{2}}^{+}C{{l}^{-}}\]) benzene diazonium hydrogen sulphate( \[{{C}_{6}}{{H}_{6}}-N_{_{2}}^{+}HSO_{4}^{-}\]) etc. are some of the examples of the diazonium salt that we should remember properly.

We here use aniline \[{{C}_{6}}{{H}_{6}}-N{{H}_{2}}\] and sodium nitrite with hydrochloric for conversion into benzenediazonium salt,

So from the above reaction we can say that we use the Gatterman reaction for the conversion of benzenediazonium to chlorobenzene or bromobenzene.
So our correct option is option C.
Note: We should also know about other examples of Gatterman reaction.

To introduce an aldehyde group to the benzene ring, Gattermann reaction is important in introducing this.
Now we will know about the difference between Sandmeyer reaction and Gatterman reaction. We should know that the Sandmeyer reaction is a type of organic substitution reaction in which we can synthesize aryl halides from aryl diazonium salts while Gattermann reaction is an organic substitution reaction in which we can formulate aromatic compounds. So, the key difference between Sandmeyer reaction and Gattermann reaction is that the Sandmeyer reaction refers to the synthesis of aryl halides from aryl diazonium salts in the presence of copper salts as a catalyst, whereas Gattermann reaction refers to the formylation of aromatic compounds in the presence of a Lewis acid catalyst.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




