
When glycerol is treated with excess of HI, it produces
a.) Allyl iodide
b.) Propene
c.) Glycerol tri iodide
d.) 2-iodopropane
Answer
531.3k+ views
Hint: The molecule of glycerol reacts with 3 HI molecules and forms an unstable compound, this unstable molecule will lose a molecule of iodine to and produces the product.
Complete step by step answer:
Glycerol is a trihydric alcohol and the IUPAC name of glycerol is propane-1,2,3-triol and in the industrial purpose it is also known as glycerin. At first glycerol or glycerin is reduced to its unstable form propene, by the formation of an unstable intermediate which is 1,2,3-triiodopropane.
HI or hydro iodic acid is generally use to prepare iodides by reaction with metal oxides or metal halides or metal hydroxides etc.
Glycerol when treated with excess HI produces 2-iodopropane.The same product will be formed when glycerol is treated with phosphorus triiodide. Glycerol reacts with 3 molecules of HI under warm conditions and produces an unstable molecule of 1,2,3-triiodopropane. This 1,2,3-triiodopropane lose a molecule of iodine and produce an alkyl iodide.
After this the alkyl iodide combines with 1 molecule of HI and produces an unstable molecule which converts into propene by losing one molecule of iodine. Propene then in presence of warn conditions converts into 2-iodopropane by addition of 1 molecule of HI.
Hence the correct answer is (D) i.e. glycerol is treated with excess of HI to produce 2-iodopropane.
So, the correct answer is “Option D”.
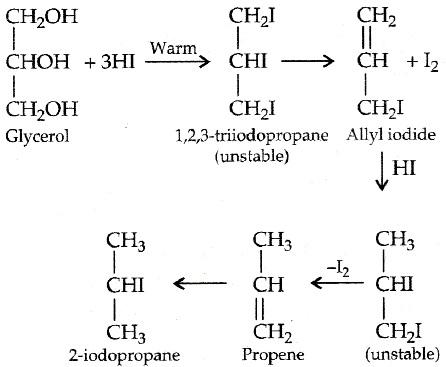
Note: While writing a chemical reaction it is important to note down the condition mentioned as it plays an important role in the mechanism of the reaction, the above reaction carried out at warm conditions. The same product will be formed when glycerol is treated with phosphorus triiodide.
Complete step by step answer:
Glycerol is a trihydric alcohol and the IUPAC name of glycerol is propane-1,2,3-triol and in the industrial purpose it is also known as glycerin. At first glycerol or glycerin is reduced to its unstable form propene, by the formation of an unstable intermediate which is 1,2,3-triiodopropane.
HI or hydro iodic acid is generally use to prepare iodides by reaction with metal oxides or metal halides or metal hydroxides etc.
Glycerol when treated with excess HI produces 2-iodopropane.The same product will be formed when glycerol is treated with phosphorus triiodide. Glycerol reacts with 3 molecules of HI under warm conditions and produces an unstable molecule of 1,2,3-triiodopropane. This 1,2,3-triiodopropane lose a molecule of iodine and produce an alkyl iodide.
After this the alkyl iodide combines with 1 molecule of HI and produces an unstable molecule which converts into propene by losing one molecule of iodine. Propene then in presence of warn conditions converts into 2-iodopropane by addition of 1 molecule of HI.
Hence the correct answer is (D) i.e. glycerol is treated with excess of HI to produce 2-iodopropane.
So, the correct answer is “Option D”.

Note: While writing a chemical reaction it is important to note down the condition mentioned as it plays an important role in the mechanism of the reaction, the above reaction carried out at warm conditions. The same product will be formed when glycerol is treated with phosphorus triiodide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




