
Graphite is a soft lubricant extremely difficult to melt. The reason for this anomalous behavior is that graphite:
A. Is an allotropic form of carbon
B. Is a non-crystalline substance
C. Has carbon atoms arranged in a large plates of rings of strongly bonded carbon, atoms with weak interplate bonds
D. Has molecules of variable molecular masses like polymers.
Answer
597.9k+ views
Hint: “Graphite has sheet structure in which different layers of graphite are arranged over each other. These layers are held together by weak Vander waals force of attractive forces, so they are free to slide over one another". That is why graphite is soft.
Complete step by step answer:
- In the given question it was mentioned that Graphite is a soft lubricant extremely difficult to melt.
- The structure of the graphite is as follows.
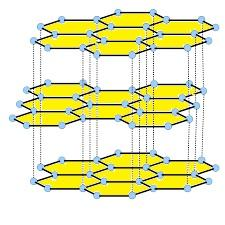
- Coming to given options, option A, Is an allotropic form of carbon. The anomalous behavior of graphite is not because of its allotropic nature with carbon.
- So, option A is not the correct answer.
- Coming option B, Is a non-crystalline substance. Because of non-crystalline structure anomalous behavior is not suitable. So, option B is not the correct answer.
- Coming to option D, which has molecules of variable molecular masses like polymers, it is also not correct. So, option d is also not correct.
- Coming to option C, it has carbon atoms arranged in a large plate of rings of strongly bonded carbon, atoms with weak interplate bonds. Yes because of this property graphite is soft lubricant extremely difficult to melt.
So, the correct option is C.
Note: Different layers of graphite are arranged over each other with weak Vander waal forces attraction. Graphite sheets move over one another because of these Vander waal forces.
Complete step by step answer:
- In the given question it was mentioned that Graphite is a soft lubricant extremely difficult to melt.
- The structure of the graphite is as follows.

- Coming to given options, option A, Is an allotropic form of carbon. The anomalous behavior of graphite is not because of its allotropic nature with carbon.
- So, option A is not the correct answer.
- Coming option B, Is a non-crystalline substance. Because of non-crystalline structure anomalous behavior is not suitable. So, option B is not the correct answer.
- Coming to option D, which has molecules of variable molecular masses like polymers, it is also not correct. So, option d is also not correct.
- Coming to option C, it has carbon atoms arranged in a large plate of rings of strongly bonded carbon, atoms with weak interplate bonds. Yes because of this property graphite is soft lubricant extremely difficult to melt.
So, the correct option is C.
Note: Different layers of graphite are arranged over each other with weak Vander waal forces attraction. Graphite sheets move over one another because of these Vander waal forces.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE





