
How can you identify optical isomers?
Answer
548.4k+ views
Hint: To identify the optical isomers, one should have the knowledge of optical isomerism and optical isomers. The isomers are those compounds which have the same molecular formula and number of atoms but differ in the arrangement of atoms.
Complete step by step answer:
Optical isomerism is defined as the type of isomerism where the isomers have identical molecular weight whereas the chemical and physical properties are also same. But, they differ in their effect on the rotation of polarized light.
Optical isomerism is seen mainly on the substances that have the same molecular and structural formula, but they cannot be superimposed on each other. In other words, we can say that they are mirror images of each other or non-super imposable mirror image of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
Typically, optical isomerism is shown by stereoisomers which rotate the plane of polarized light. If the plane of polarized light passing through enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as (+) form and if the plane of polarized light rotates in anti-clockwise direction then the enantiomer is said to exist in (-). This means that the optical isomers are optically active.
Optical isomers are two compounds which contain the same number and kinds of atoms, and bonds but differ in the arrangement of the atoms. Each non-superimposable mirror image structure is called an enantiomer. Molecules or ions that are present in optical isomers are called chiral.
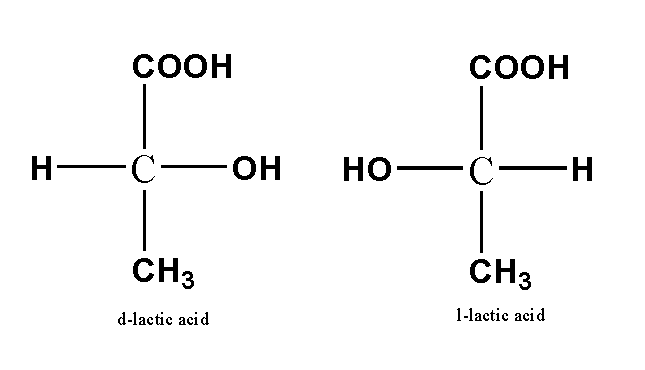
Examples of optical isomers are shown below.
Note:
The chiral compound means that the chiral carbon is attached to four different atoms or the group of atoms that is why the optical isomers are asymmetric in nature.
Complete step by step answer:
Optical isomerism is defined as the type of isomerism where the isomers have identical molecular weight whereas the chemical and physical properties are also same. But, they differ in their effect on the rotation of polarized light.
Optical isomerism is seen mainly on the substances that have the same molecular and structural formula, but they cannot be superimposed on each other. In other words, we can say that they are mirror images of each other or non-super imposable mirror image of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
Typically, optical isomerism is shown by stereoisomers which rotate the plane of polarized light. If the plane of polarized light passing through enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as (+) form and if the plane of polarized light rotates in anti-clockwise direction then the enantiomer is said to exist in (-). This means that the optical isomers are optically active.
Optical isomers are two compounds which contain the same number and kinds of atoms, and bonds but differ in the arrangement of the atoms. Each non-superimposable mirror image structure is called an enantiomer. Molecules or ions that are present in optical isomers are called chiral.
Examples of optical isomers are shown below.

Note:
The chiral compound means that the chiral carbon is attached to four different atoms or the group of atoms that is why the optical isomers are asymmetric in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




