
Identify A
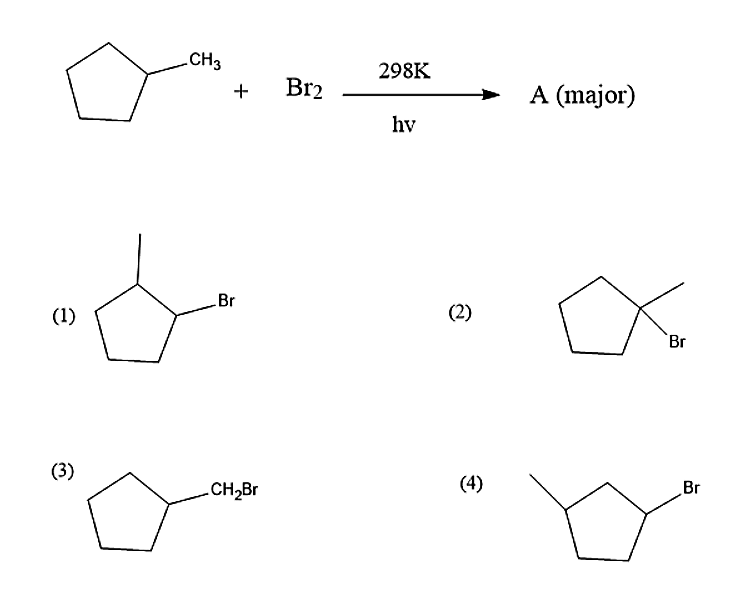

Answer
569.7k+ views
Hint: 1-Methyl cyclopentane reacts with Bromine molecule in presence of UV light. Such a reaction is called a free radical reaction. Here at first bromine will become bromine free radical and then after that, it would attack desired carbon to give desired product.
Complete Solution :
Cycloalkane when it reacts with bromine molecules in the presence of sunlight or UV light, it undergoes free radical substitution reaction. Free radical substitution reaction can take place in three steps, namely:
- Initiation
- Propagation
- Termination
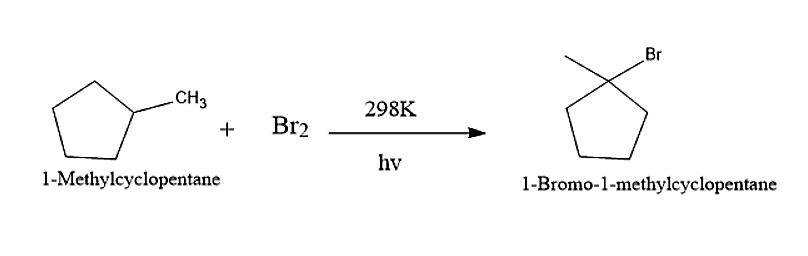
Now let us consider 1-methyl cyclopentane
When 1-Methyl cyclopentane reacts with Bromine molecules in presence of UV-light, then the major product obtained will be 1-Bromo-1-methylcyclopentane.
The reaction is given below:
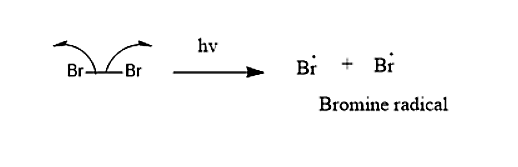
Mechanism which is taking place in this reaction will be
Step 1: Bromine molecule split into free-radical in presence of the UV-light.
Step2: Formation of the most stable product.
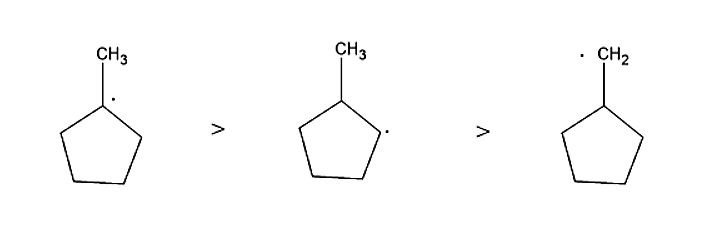
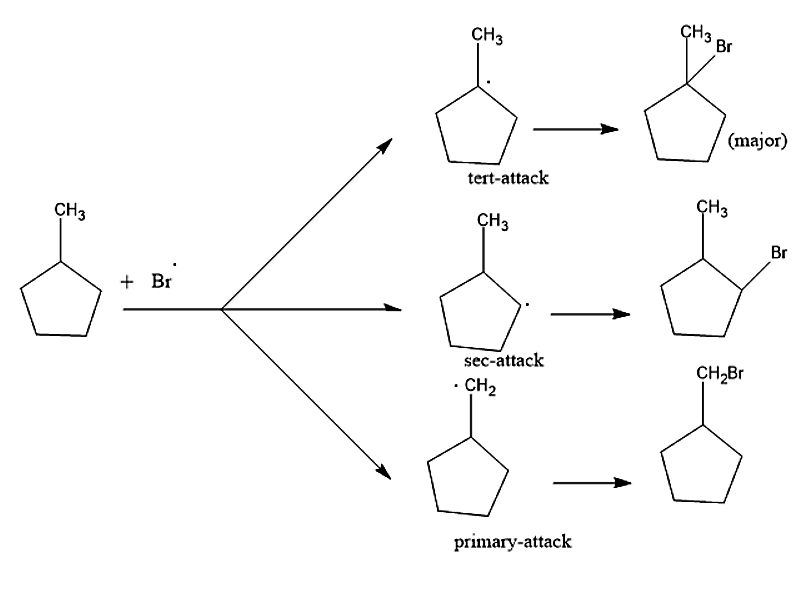 Bromine when it attacks 1-Methyl cyclopentane in presence of UV light, it forms the stable product by attaching itself with carbon containing least number of hydrogen atoms or carbon which is most substituted. Hence the major product formed will be 1-Bromo-1- methyl cyclopentane.
Bromine when it attacks 1-Methyl cyclopentane in presence of UV light, it forms the stable product by attaching itself with carbon containing least number of hydrogen atoms or carbon which is most substituted. Hence the major product formed will be 1-Bromo-1- methyl cyclopentane.
Hence the correct answer is option (2) 1-Bromo-1- methyl cyclopentane.
Additional Information.
The free radical play important role in the following reactions such as
- Combustion reaction.
- Polymerization
- plasma chemistry
- Atmospheric chemistry
- Biochemistry.
Note: The reactivity order of the carbocations are given in the following order:
\[3^\circ > 2^\circ > 1^\circ \]
Complete Solution :
Cycloalkane when it reacts with bromine molecules in the presence of sunlight or UV light, it undergoes free radical substitution reaction. Free radical substitution reaction can take place in three steps, namely:
- Initiation
- Propagation
- Termination
Now let us consider 1-methyl cyclopentane
When 1-Methyl cyclopentane reacts with Bromine molecules in presence of UV-light, then the major product obtained will be 1-Bromo-1-methylcyclopentane.
The reaction is given below:

Mechanism which is taking place in this reaction will be
Step 1: Bromine molecule split into free-radical in presence of the UV-light.

Step2: Formation of the most stable product.

Hence the correct answer is option (2) 1-Bromo-1- methyl cyclopentane.
Additional Information.
The free radical play important role in the following reactions such as
- Combustion reaction.
- Polymerization
- plasma chemistry
- Atmospheric chemistry
- Biochemistry.
Note: The reactivity order of the carbocations are given in the following order:
\[3^\circ > 2^\circ > 1^\circ \]

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




