
Identify the order of acidic strength of the following molecules.
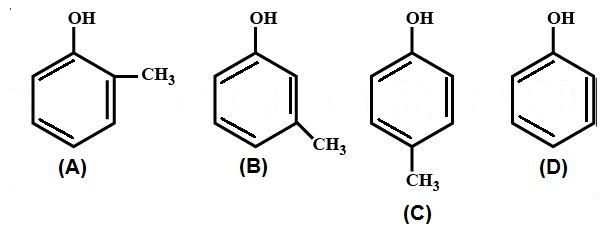
A.A > B > C > D
B.D > C > B > A
C.D > B > C > A
D.A > B > D > C

Answer
583.8k+ views
Hint:Use the inductive effect, which is the effect of transfer of unequal sharing of electrons which are bonded with a chain of atoms and create permanent dipole in that molecule.
The inductive effect decreases the acidic character if any group shows inductive effect of $ + I$. The group $ - C{H_3}$ shows inductive effect of $ + I$.
Complete step by step answer:
When the unequal sharing of electrons which are bonded in a molecule there arises the permanent dipole. Then, this phenomenon is known as the inductive effect. This effect arises in sigma bonds.
By using the inductive effect, we can determine the acidic and basic character of the molecule by the help of an electron donating group which decreases the acidity of the molecule and an electron withdrawing group which increases the acidic character of the molecule.
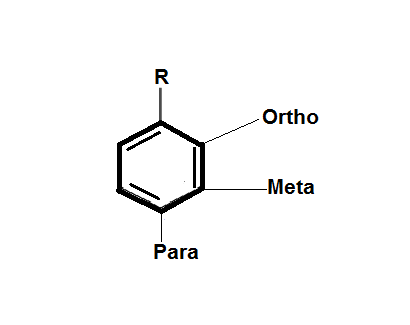
Here, if R is the electron withdrawing group then, the conjugate base is stabilized via delocalization of the formed negative charge and if R is the electron donating group, then due to the inter electronic repulsions the conjugate base will destabilize.
So, the acidic character of groups which have inductive effect as $ + I$ will decrease and the acidic character of groups having inductive effect as $ - I$ will increase.
Now, according to the question, the $ - C{H_3}$ shows inductive effect $ + I$ which decreases the acidic character of the molecule. When the $ - C{H_3}$ is at ortho position it shows greater $ + I$ inductive effect than meta and para positions. Therefore, the correct order of acidity is D > C > B > A.
Hence, the correct option is (B).
Note:The positive inductive effect or $ + I$ effect can be defined as the chemical species with their abilities to release the electrons then, the charge is relayed to the chain and there arises the positive inductive effect.The acidic character of the molecule can be determined by $Ka$ of acid and $pKa$ of acid.
The inductive effect decreases the acidic character if any group shows inductive effect of $ + I$. The group $ - C{H_3}$ shows inductive effect of $ + I$.
Complete step by step answer:
When the unequal sharing of electrons which are bonded in a molecule there arises the permanent dipole. Then, this phenomenon is known as the inductive effect. This effect arises in sigma bonds.
By using the inductive effect, we can determine the acidic and basic character of the molecule by the help of an electron donating group which decreases the acidity of the molecule and an electron withdrawing group which increases the acidic character of the molecule.

Here, if R is the electron withdrawing group then, the conjugate base is stabilized via delocalization of the formed negative charge and if R is the electron donating group, then due to the inter electronic repulsions the conjugate base will destabilize.
So, the acidic character of groups which have inductive effect as $ + I$ will decrease and the acidic character of groups having inductive effect as $ - I$ will increase.
Now, according to the question, the $ - C{H_3}$ shows inductive effect $ + I$ which decreases the acidic character of the molecule. When the $ - C{H_3}$ is at ortho position it shows greater $ + I$ inductive effect than meta and para positions. Therefore, the correct order of acidity is D > C > B > A.
Hence, the correct option is (B).
Note:The positive inductive effect or $ + I$ effect can be defined as the chemical species with their abilities to release the electrons then, the charge is relayed to the chain and there arises the positive inductive effect.The acidic character of the molecule can be determined by $Ka$ of acid and $pKa$ of acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




