
If the boiling point of ethanol (molecular weight= 46) is $ 78{}^\circ \text{C} $ , what is the boiling point of diethyl ether (molecular weight=74)?
(A) $ 100{}^\circ \text{C} $
(B) $ 70{}^\circ \text{C} $
(C) $ \text{86}{}^\circ \text{C} $
(D) $ 34{}^\circ \text{C} $
Answer
552k+ views
Hint: Boiling point of any liquid is defined as the point at which liquid starts to boil and changes into vapour phase. With increase in the molecular weight the boiling point also increases.
Boiling point of liquid depends on the various factors like molecular weight, hydrogen bonding etc.
Complete step by step solution
Structure of diethyl ether $ =\text{C}{{\text{H}}_{3}}\text{- }6{{\text{H}}_{2}}\text{- O - C}{{\text{H}}_{3}}\text{ - C}{{\text{H}}_{2}} $
Molecular weight of diethyl ether is = 74
Structure of ethanol $ =\text{C}{{\text{H}}_{3}}\text{- C}{{\text{H}}_{2}}\text{ - OH} $
Molecular weight is = 46 g
As we know that with increase in molecular weight boiling point also increases. But in case of diethyl ether and ethanol this is not true, because strong H- bonding is present in ethanol due to which boiling point of ethanol is greater than diethyl ether.
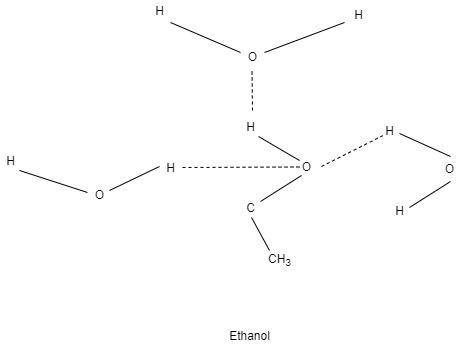
Boiling point of diethyl ether is about $ 34{}^\circ \text{C} $
So, Option (D) is correct.
Note
To understand this kind of problem we should know the structure and boiling point trends. Also one has to remember some exceptional cases or effects of H- binding on the boiling point. Ethanol is an organic compound which is also known as ethyl alcohol. It is a colourless alcohol. The boiling point of ethanol is lower than that of water. In diethyl ether, oxygen atoms are linked to two ethyl groups. It is a non-polar solvent and is used as a refrigerant. It is colourless and highly volatile in nature. It is sweet smelling in nature.
Boiling point of liquid depends on the various factors like molecular weight, hydrogen bonding etc.
Complete step by step solution
Structure of diethyl ether $ =\text{C}{{\text{H}}_{3}}\text{- }6{{\text{H}}_{2}}\text{- O - C}{{\text{H}}_{3}}\text{ - C}{{\text{H}}_{2}} $
Molecular weight of diethyl ether is = 74
Structure of ethanol $ =\text{C}{{\text{H}}_{3}}\text{- C}{{\text{H}}_{2}}\text{ - OH} $
Molecular weight is = 46 g
As we know that with increase in molecular weight boiling point also increases. But in case of diethyl ether and ethanol this is not true, because strong H- bonding is present in ethanol due to which boiling point of ethanol is greater than diethyl ether.

Boiling point of diethyl ether is about $ 34{}^\circ \text{C} $
So, Option (D) is correct.
Note
To understand this kind of problem we should know the structure and boiling point trends. Also one has to remember some exceptional cases or effects of H- binding on the boiling point. Ethanol is an organic compound which is also known as ethyl alcohol. It is a colourless alcohol. The boiling point of ethanol is lower than that of water. In diethyl ether, oxygen atoms are linked to two ethyl groups. It is a non-polar solvent and is used as a refrigerant. It is colourless and highly volatile in nature. It is sweet smelling in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




