
In antifluorite structure, coordination number of anion is:
a.) 4
b.) 6
c.) 8
d.) 12
Answer
601.2k+ views
Hint: Antiflourite has FCC packing of the anions in which all the tetrahedral sites are filled by cations.
The face-centred cubic (fcc) structure has a coordination number of 12 and contains 4 atoms per unit cell. These two facts can be used to deduce the answer for the question considering the fact that antifluorite has FCC packing which is already given in the question.
Complete step-by-step answer:
Face Centred Cubic Structure consists of an atom placed at each cube corner that is all 8 corners of the cubic structure and an atom in the centre of all the six faces of the cube. A unit cell is considered to be the smallest representation of an entire crystal. And thus, all crystal lattices are built of repeating unit cells.
In a unit cell, an atom's coordination number is the number of atoms it is in contact with.
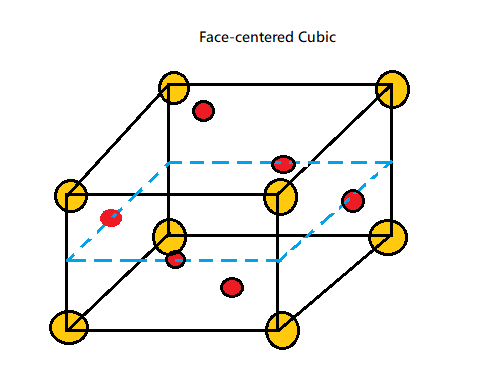
The coordination number is the number of atoms that surround a central atom in a crystal lattice arrangement. It is also known as Ligancy.
Chemical formula for the Antiflourite is ${M_2}X$, Example: $L{i_2}O,N{a_2}O,{K_2}O$
The Radius ratio (rc/ra) is 0.225:0.414 in case of the Antifluorite structure.
In the FCC packed structure of antifluorite in many cases, even though rc/ra ratio predicts an octahedral coordination, yet tetrahedral coordination is preferred to fulfil the stoichiometry requirements. Which results in the fact that anions are cubic coordinated by the cations.
As in case of the Antifluorite structure, it has FCC packing of anions.
All tetrahedral sites are filled with cations so which clearly implies that coordination number of cations is 4.
And since the coordination number of FCC structure is 12, therefore it implies clearly that the coordination number of anions is 8.
Therefore, the correct option for the above question is – Option (A) i.e. 4
Note – Antifluorite structure has the opposite arrangement of ions than the fluorite structure. The space efficiency of the Face Centred cubic structure is 74.04% .
The face-centred cubic (fcc) structure has a coordination number of 12 and contains 4 atoms per unit cell. These two facts can be used to deduce the answer for the question considering the fact that antifluorite has FCC packing which is already given in the question.
Complete step-by-step answer:
Face Centred Cubic Structure consists of an atom placed at each cube corner that is all 8 corners of the cubic structure and an atom in the centre of all the six faces of the cube. A unit cell is considered to be the smallest representation of an entire crystal. And thus, all crystal lattices are built of repeating unit cells.
In a unit cell, an atom's coordination number is the number of atoms it is in contact with.

The coordination number is the number of atoms that surround a central atom in a crystal lattice arrangement. It is also known as Ligancy.
Chemical formula for the Antiflourite is ${M_2}X$, Example: $L{i_2}O,N{a_2}O,{K_2}O$
The Radius ratio (rc/ra) is 0.225:0.414 in case of the Antifluorite structure.
In the FCC packed structure of antifluorite in many cases, even though rc/ra ratio predicts an octahedral coordination, yet tetrahedral coordination is preferred to fulfil the stoichiometry requirements. Which results in the fact that anions are cubic coordinated by the cations.
As in case of the Antifluorite structure, it has FCC packing of anions.
All tetrahedral sites are filled with cations so which clearly implies that coordination number of cations is 4.
And since the coordination number of FCC structure is 12, therefore it implies clearly that the coordination number of anions is 8.
Therefore, the correct option for the above question is – Option (A) i.e. 4
Note – Antifluorite structure has the opposite arrangement of ions than the fluorite structure. The space efficiency of the Face Centred cubic structure is 74.04% .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




