
In benzyne, the triple bond consists of:
A. One $sp-sp$ $\sigma $- bond and two $p-p$ $\pi $- bonds
B. Two $sp-sp$ $\sigma $- bonds and one $p-p$ $\pi $- bond
C. One $s{{p}^{2}}-s{{p}^{2}}$ $\sigma $- bond and one $p-p$ $\pi $- bond
D. One $s{{p}^{2}}-s{{p}^{2}}$ $\sigma $- bond, one $s{{p}^{2}}-s{{p}^{2}}$ $\pi $- bond, and one $p-p$ $\pi $- bond.
Answer
569.7k+ views
Hint: Try to recall that in benzyne all the carbon atoms are $s{{p}^{2}}$ hybridized and one $\pi $- bond is formed by hybridized orbitals. Now, by using this you can easily find the correct option from the given ones.
Complete step by step answer:
- It is known to you that benzyne is a neutral, highly intermediate in which aromatic characters are not markedly distributed.
- The benzyne contains a triple bond which is not like the triple bond of acetylene where the two carbon atoms form a $\sigma $- bond using $sp$ orbitals and the remaining are used to form $p-p$ $\pi $- bonds.
- Such a structure like acetylene triple bond is not possible in benzyne because of the hexagonal geometry associated with benzene ring. Most probably the new mount of benzene is formed by the sideways overlapping of $s{{p}^{2}}$orbitals of the two adjacent carbon atoms. So, the triple bond contains one $s{{p}^{2}}-s{{p}^{2}}$ $\sigma $- bond, one $s{{p}^{2}}-s{{p}^{2}}$ $\pi $- bond and one $p-p$ $\pi $- bond.
- This new bond orbitals lie along the side of the ring, and has little interaction with the pi cloud lying above and below the ring. Since the sideways overlapping is not very good, the new bond is weaker one and hence benzyne is highly reactive.
- The structure of benzyne is given below:
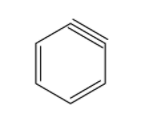
Therefore, from above we can conclude that option D is the correct option to the given question.
Note: Note that benzyne is formed from benzene via a free radical elimination reaction in which the pi-bond between the two carbon atoms is formed by $s{{p}^{2}}$ hybridized orbitals. This is the only case in which a pi-bond is formed by hybridized orbitals.
Complete step by step answer:
- It is known to you that benzyne is a neutral, highly intermediate in which aromatic characters are not markedly distributed.
- The benzyne contains a triple bond which is not like the triple bond of acetylene where the two carbon atoms form a $\sigma $- bond using $sp$ orbitals and the remaining are used to form $p-p$ $\pi $- bonds.
- Such a structure like acetylene triple bond is not possible in benzyne because of the hexagonal geometry associated with benzene ring. Most probably the new mount of benzene is formed by the sideways overlapping of $s{{p}^{2}}$orbitals of the two adjacent carbon atoms. So, the triple bond contains one $s{{p}^{2}}-s{{p}^{2}}$ $\sigma $- bond, one $s{{p}^{2}}-s{{p}^{2}}$ $\pi $- bond and one $p-p$ $\pi $- bond.
- This new bond orbitals lie along the side of the ring, and has little interaction with the pi cloud lying above and below the ring. Since the sideways overlapping is not very good, the new bond is weaker one and hence benzyne is highly reactive.
- The structure of benzyne is given below:

Therefore, from above we can conclude that option D is the correct option to the given question.
Note: Note that benzyne is formed from benzene via a free radical elimination reaction in which the pi-bond between the two carbon atoms is formed by $s{{p}^{2}}$ hybridized orbitals. This is the only case in which a pi-bond is formed by hybridized orbitals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




