
In gaseous reactions important for the understanding of the upper atmosphere $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O react by molecularly to form two $\text{ OH }$ radicals. $\text{ }\Delta \text{H }$ for this reaction is $\text{72 kJ }$ at 500 K and Ea is $\text{ 77 kJ mo}{{\text{l}}^{-1}}\text{ }$ , then Ea for the bimolecular recombination of two $\text{ OH }$ radicals to form $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O is:
A) $\text{ 3 kJ mo}{{\text{l}}^{-1}}$
B) $\text{ 4 kJ mo}{{\text{l}}^{-1}}$
C) $\text{ 5 kJ mo}{{\text{l}}^{-1}}$
D) $\text{ 7 kJ mo}{{\text{l}}^{-1}}$
Answer
578.4k+ views
Hint: The reaction to convert to the reactant or product either absorbs the heat or liberates the heat. The reaction in which the reactant liberates heat and forms reactant is exothermic. The reactant is converted into the product only when the molecules acquire the minimum energy to cross the energy barrier, activation energy Ea. On plot of potential energy against the reaction coordinates, for exothermic reactions the energy of the reactant is always on the high end as compared to the product. Activation energy for the backward reaction can be calculated as,
$\text{ E}{{\text{a}}_{\text{(Backward)}}}\text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta {{\text{H}}_{\text{(exothermic)}}}$
Complete answer:
Here we have given the following data:
In the gaseous state, the $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O reacts by molecularly.i.e. The rate of reaction depends on the concentration of $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O and generates the $\text{ OH }$ radical.
The enthalpy of reaction is equal to $\text{ }\Delta \text{H= - 72 kJ }$
The activation energy of the reaction is,$\text{ Ea = 77 kJ mo}{{\text{l}}^{-1}}\text{ }$
We are interested to find out the activation energy for the recombination of two $\text{ OH }$ radicals to form $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O as the product.
The reactions of formation of $\text{ OH }$ radical (forward) are shown below,
$\text{ }{{\text{H}}_{\text{2}}}\text{O + O }\to \text{ 2O}{{\text{H}}^{\bullet }}\text{ }, \Delta \text{H = }-72\text{ kJ , Ea = 77 kJ mo}{{\text{l}}^{-1}}\text{ }$
The reactions of formation of $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ an O as the product from the two $\text{ OH }$ radical is given as follows,
$\text{ 2O}{{\text{H}}^{\bullet }}\text{ }\to \text{ }{{\text{H}}_{\text{2}}}\text{O + O }$
To find out the activation energy we must consider the plot of potential energy against the reaction coordinates.
Here, the forward reaction is forming a single product that liberates the energy thus the enthalpy of the reaction is negative. It means that reactant $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O initially placed at the high heat as shown in the figure. As the reaction proceeds the $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O acquires the energy which is equal to the activation energy (or energy barrier) and transforms into the product. The $\text{ OH }$ radical formation is accompanied by the liberation of heat equal to the sum of enthalpy $\text{ }\Delta \text{H= + 72 kJ }$and activation energy .thus, the product have are stable and have low potential energy. This is summarised in the figure below,
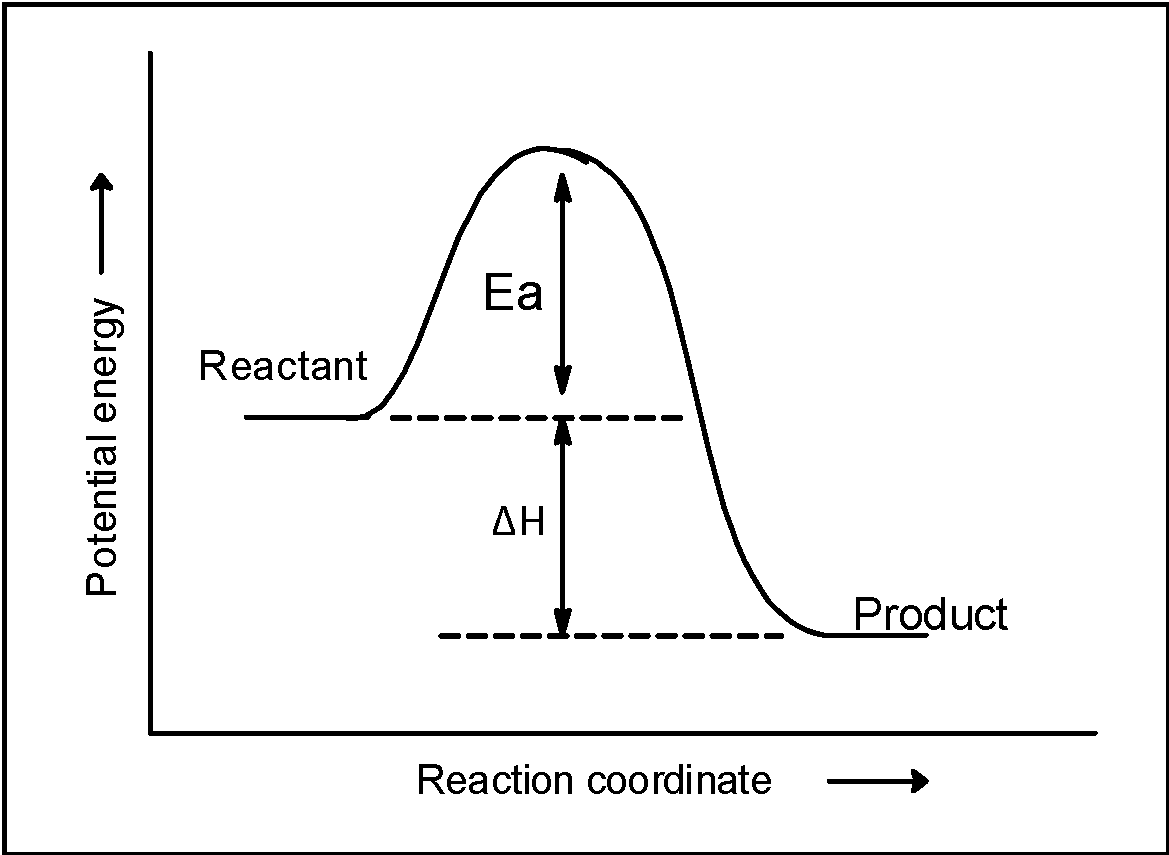
Now, from the plot, it is clear that if the product $\text{ OH }$ radical combines and goes towards the reactant then the activation energy acquired by the molecules must be equal to the sum of the activation energy of forwarding reaction and the enthalpy of reaction. This can be utilized to find the activation energy of backward reaction as follows,
$\text{ Ea }$ For the reverse reaction,
$\begin{align}
& \text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta \text{H } \\
& \text{= 77 + (}-72) \\
& \therefore \text{E}{{\text{a}}_{\text{(Backward)}}}\text{ = }~~+5\text{ kJ mo}{{\text{l}}^{-1}} \\
\end{align}$
It is the minimum amount of energy required by the $\text{ OH }$ radical to undergo the reaction to form water and oxygen atoms.
Therefore, the activation energy for the backward reaction is $5\text{ kJ mo}{{\text{l}}^{-1}}$.
Hence, (C) is the correct option.
Note:
Note that, in the above reaction the reactant liberates energy to form low energy products, thus this is an exothermic reaction and therefore we have taken the negative value of enthalpy. However, there is a certain reaction in which the product acquires a larger energy than the reactant. This is an endothermic reaction and heat change in such a reaction is considered positive. In an endothermic reaction, the activation energy for the backward reaction would be equal to,
$\text{ E}{{\text{a}}_{\text{(Backward)}}}\text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta {{\text{H}}_{\text{(endothermic)}}}$
$\text{ E}{{\text{a}}_{\text{(Backward)}}}\text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta {{\text{H}}_{\text{(exothermic)}}}$
Complete answer:
Here we have given the following data:
In the gaseous state, the $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O reacts by molecularly.i.e. The rate of reaction depends on the concentration of $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O and generates the $\text{ OH }$ radical.
The enthalpy of reaction is equal to $\text{ }\Delta \text{H= - 72 kJ }$
The activation energy of the reaction is,$\text{ Ea = 77 kJ mo}{{\text{l}}^{-1}}\text{ }$
We are interested to find out the activation energy for the recombination of two $\text{ OH }$ radicals to form $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O as the product.
The reactions of formation of $\text{ OH }$ radical (forward) are shown below,
$\text{ }{{\text{H}}_{\text{2}}}\text{O + O }\to \text{ 2O}{{\text{H}}^{\bullet }}\text{ }, \Delta \text{H = }-72\text{ kJ , Ea = 77 kJ mo}{{\text{l}}^{-1}}\text{ }$
The reactions of formation of $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ an O as the product from the two $\text{ OH }$ radical is given as follows,
$\text{ 2O}{{\text{H}}^{\bullet }}\text{ }\to \text{ }{{\text{H}}_{\text{2}}}\text{O + O }$
To find out the activation energy we must consider the plot of potential energy against the reaction coordinates.
Here, the forward reaction is forming a single product that liberates the energy thus the enthalpy of the reaction is negative. It means that reactant $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O initially placed at the high heat as shown in the figure. As the reaction proceeds the $\text{ }{{\text{H}}_{\text{2}}}\text{O}$ and O acquires the energy which is equal to the activation energy (or energy barrier) and transforms into the product. The $\text{ OH }$ radical formation is accompanied by the liberation of heat equal to the sum of enthalpy $\text{ }\Delta \text{H= + 72 kJ }$and activation energy .thus, the product have are stable and have low potential energy. This is summarised in the figure below,

Now, from the plot, it is clear that if the product $\text{ OH }$ radical combines and goes towards the reactant then the activation energy acquired by the molecules must be equal to the sum of the activation energy of forwarding reaction and the enthalpy of reaction. This can be utilized to find the activation energy of backward reaction as follows,
$\text{ Ea }$ For the reverse reaction,
$\begin{align}
& \text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta \text{H } \\
& \text{= 77 + (}-72) \\
& \therefore \text{E}{{\text{a}}_{\text{(Backward)}}}\text{ = }~~+5\text{ kJ mo}{{\text{l}}^{-1}} \\
\end{align}$
It is the minimum amount of energy required by the $\text{ OH }$ radical to undergo the reaction to form water and oxygen atoms.
Therefore, the activation energy for the backward reaction is $5\text{ kJ mo}{{\text{l}}^{-1}}$.
Hence, (C) is the correct option.
Note:
Note that, in the above reaction the reactant liberates energy to form low energy products, thus this is an exothermic reaction and therefore we have taken the negative value of enthalpy. However, there is a certain reaction in which the product acquires a larger energy than the reactant. This is an endothermic reaction and heat change in such a reaction is considered positive. In an endothermic reaction, the activation energy for the backward reaction would be equal to,
$\text{ E}{{\text{a}}_{\text{(Backward)}}}\text{ = E}{{\text{a}}_{\text{(forward)}}}\text{ + }\Delta {{\text{H}}_{\text{(endothermic)}}}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




