
In the following sequence of reactions: Toluene$\xrightarrow{{KMn{O_4}}}$A$\xrightarrow{{SOC{l_2}}}$B$\xrightarrow[{BaS{O_4}}]{{{H_2}/Pd}}$C; then product C is:
(A) ${C_6}{H_5}COOH$
(B) ${C_6}{H_5}C{H_3}$
(C) ${C_6}{H_5}C{H_2}OH$
(D) ${C_6}{H_5}CHO$
Answer
579.3k+ views
Hint: We should know that $KMn{O_4}$ is an oxidising agent, $SOC{l_2}$ is an acylating agent and ${H_2}/Pd$ or $BaS{O_4}$ are reducing agents which cause the Rosenmund’s reduction.
Complete step by step answer:
-In this question we need to go step by step for each reactant and first find A, then B and then C as shown in the reaction: Toluene$\xrightarrow{{KMn{O_4}}}$A$\xrightarrow{{SOC{l_2}}}$B$\xrightarrow[{BaS{O_4}}]{{{H_2}/Pd}}$C
-Now let us begin by finding (A). We know that the initial reactant is toluene (${C_6}{H_5} - C{H_3}$) and $KMn{O_4}$ is a strong oxidizing agent and it oxidizes toluene to benzoic acid. The reaction is shown below:
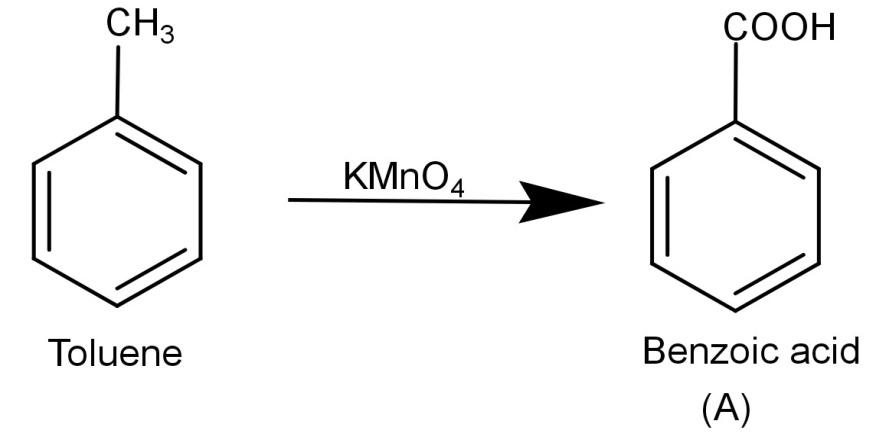
-Now we need to react (A) Benzoic acid with $SOC{l_2}$ (Thionyl chloride). The thionyl chloride converts carboxylic acid to acid chloride and hence it will convert benzoic acid to benzoyl chloride. The reaction is shown below:
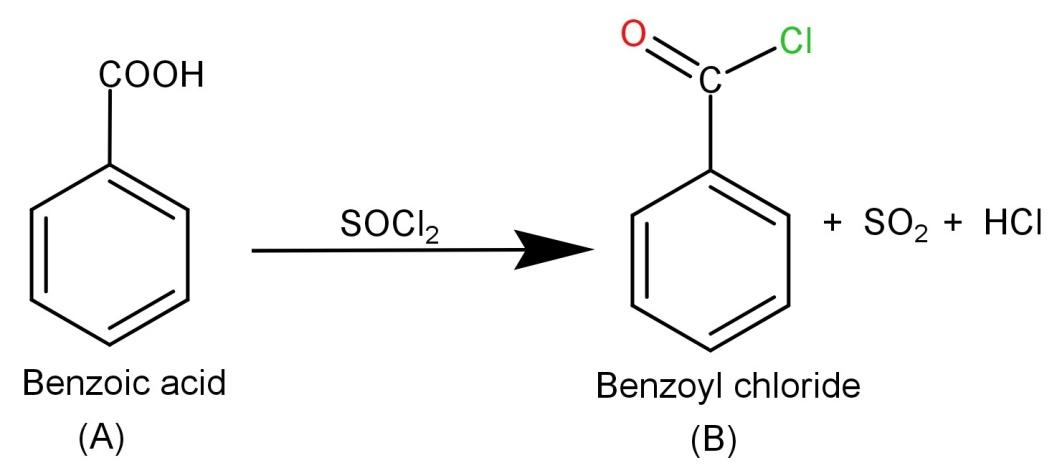
-Finally the product (B) Benzoyl chloride needs to be reacted with ${H_2}/Pd$ or $BaS{O_4}$. They are reducing agents and hence Rosenmund reduction occurs which causes the conversion of benzoyl chloride to Benzaldehyde. So the final product is Benzaldehyde and the involved reaction is:
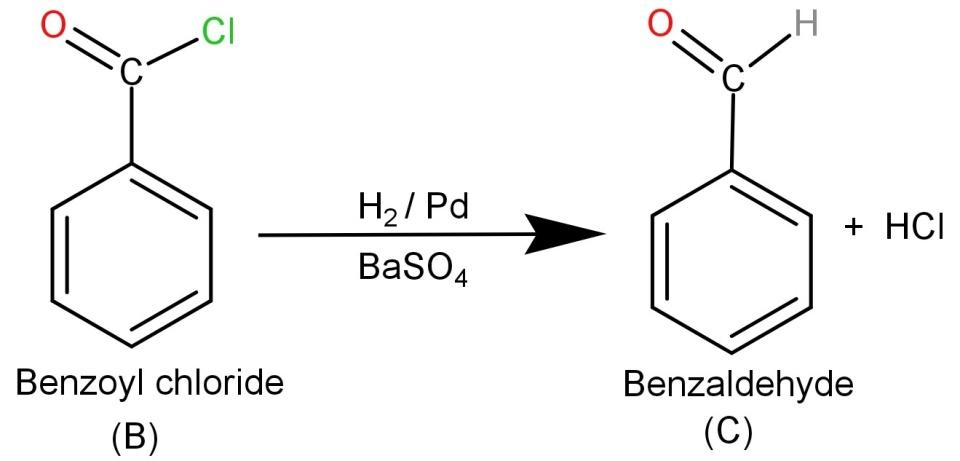
From this we know that (C) is Benzaldehyde (${C_6}{H_5}CHO$)
So, the correct answer is “Option D”.
Note: Since Benzaldehyde is a colourless liquid with a characteristic almond like odour, it can be used as a bitter component of almond oil and is also used to impart artificial almond flavour to foods and some other scented products.
Complete step by step answer:
-In this question we need to go step by step for each reactant and first find A, then B and then C as shown in the reaction: Toluene$\xrightarrow{{KMn{O_4}}}$A$\xrightarrow{{SOC{l_2}}}$B$\xrightarrow[{BaS{O_4}}]{{{H_2}/Pd}}$C
-Now let us begin by finding (A). We know that the initial reactant is toluene (${C_6}{H_5} - C{H_3}$) and $KMn{O_4}$ is a strong oxidizing agent and it oxidizes toluene to benzoic acid. The reaction is shown below:

-Now we need to react (A) Benzoic acid with $SOC{l_2}$ (Thionyl chloride). The thionyl chloride converts carboxylic acid to acid chloride and hence it will convert benzoic acid to benzoyl chloride. The reaction is shown below:

-Finally the product (B) Benzoyl chloride needs to be reacted with ${H_2}/Pd$ or $BaS{O_4}$. They are reducing agents and hence Rosenmund reduction occurs which causes the conversion of benzoyl chloride to Benzaldehyde. So the final product is Benzaldehyde and the involved reaction is:

From this we know that (C) is Benzaldehyde (${C_6}{H_5}CHO$)
So, the correct answer is “Option D”.
Note: Since Benzaldehyde is a colourless liquid with a characteristic almond like odour, it can be used as a bitter component of almond oil and is also used to impart artificial almond flavour to foods and some other scented products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




