
Iodoform reaction is answered by all except:
[A] $C{{H}_{3}}CHO$
[B] $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$
[C] $C{{H}_{3}}CHOHC{{H}_{2}}COOH$
[D] $C{{H}_{3}}C{{H}_{2}}OH$
Answer
593.7k+ views
Hint: Iodoform is generally shown by ketones, aldehyde and also alcohol. The only aldehyde which shows positive results in iodoform test is acetaldehyde and the only alcohol which shows positive results is ethanol.
Complete step by step answer:
Iodoform test is used in laboratories for the detection of aldehydes and ketones containing an alpha methyl group.
Compounds containing $C{{H}_{3}}CO$ or $C{{H}_{3}}CH(OH)$ groups show positive results in iodoform test.
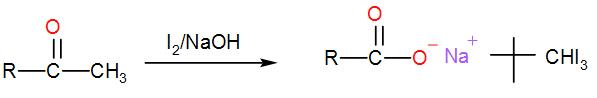
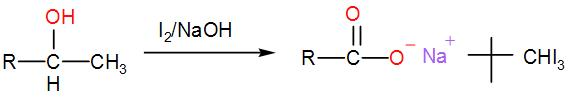
When iodine and sodium hydroxide are added to a compound containing methyl ketone or a secondary alcohol having a methyl group in alpha position, a yellow iodine precipitate is obtained due to formation of triiodomethane. The reaction is:
Now, let us check which of the given options show iodoform test;
In option [D], we have $C{{H}_{3}}C{{H}_{2}}OH$
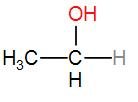
It is ethanol and it contains a $C{{H}_{3}}CH(OH)$ group. Therefore iodoform will be answered by it.
In option [C], we have $C{{H}_{3}}CHOHC{{H}_{2}}COOH$
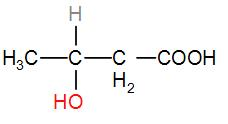
It has a $C{{H}_{3}}CH(OH)$ group, therefore the iodoform test will be answered by it.
In option [B], we have $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$
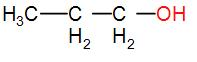
It does not have either $C{{H}_{3}}CO$ nor $C{{H}_{3}}CH(OH)$ group. Therefore, it will not show the iodoform test.
Lastly, in option [A] we have, $C{{H}_{3}}CHO$
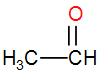
It has a $C{{H}_{3}}CO$ group, therefore it will show iodoform test too.
Therefore, the only compound which will not show positive result in iodoform test is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$.
Therefore, the correct answer is option [B] $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$
Note:
Iodoform test is used to detect ethanol or ethanal (acetaldehyde) as they are the only alcohol and aldehyde respectively which show the iodoform test. Solution to such questions can be easily found just by drawing the structure but we have to remember that this test is shown only by the compounds containing $C{{H}_{3}}CO$ or $C{{H}_{3}}CH(OH)$ group.
Complete step by step answer:
Iodoform test is used in laboratories for the detection of aldehydes and ketones containing an alpha methyl group.
Compounds containing $C{{H}_{3}}CO$ or $C{{H}_{3}}CH(OH)$ groups show positive results in iodoform test.
When iodine and sodium hydroxide are added to a compound containing methyl ketone or a secondary alcohol having a methyl group in alpha position, a yellow iodine precipitate is obtained due to formation of triiodomethane. The reaction is:


Now, let us check which of the given options show iodoform test;
In option [D], we have $C{{H}_{3}}C{{H}_{2}}OH$

It is ethanol and it contains a $C{{H}_{3}}CH(OH)$ group. Therefore iodoform will be answered by it.
In option [C], we have $C{{H}_{3}}CHOHC{{H}_{2}}COOH$

It has a $C{{H}_{3}}CH(OH)$ group, therefore the iodoform test will be answered by it.
In option [B], we have $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$
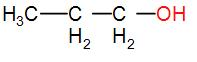
It does not have either $C{{H}_{3}}CO$ nor $C{{H}_{3}}CH(OH)$ group. Therefore, it will not show the iodoform test.
Lastly, in option [A] we have, $C{{H}_{3}}CHO$

It has a $C{{H}_{3}}CO$ group, therefore it will show iodoform test too.
Therefore, the only compound which will not show positive result in iodoform test is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$.
Therefore, the correct answer is option [B] $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH$
Note:
Iodoform test is used to detect ethanol or ethanal (acetaldehyde) as they are the only alcohol and aldehyde respectively which show the iodoform test. Solution to such questions can be easily found just by drawing the structure but we have to remember that this test is shown only by the compounds containing $C{{H}_{3}}CO$ or $C{{H}_{3}}CH(OH)$ group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




