
Iodoform reaction is given by:
(A) Acetaldehyde
(B) Methyl alcohol
(C) Formaldehyde
(D) Formic acid
Answer
587.7k+ views
Hint: The iodoform test is a highly useful method which can be used to detect the presence of carbonyl groups or methyl ketone groups in an unknown compound. In the test a solution of Iodine is added to a small amount of our unknown substance, followed by sodium hydroxide. The formation of triiodomethane can be treated as a positive result.
Complete step by step answer:
- The iodoform test is a test which is used to detect the presence of alcohols with the structure $RCH\left( OH \right)C{{H}_{3}}$ and carbonyl compounds with the structure $RCOC{{H}_{3}}$ . Iodoform is a chemical compound triiodomethane with the chemical formula $CH{{I}_{3}}$ .
- There are two different ways in which we can perform the iodoform reaction. One is by using iodine and sodium hydroxide solution and the other one is by using potassium iodide and sodium hypochlorite solutions.
- If the reaction result is positive or in other words the reactants contain the above-mentioned compounds, then a pale-yellow precipitate with the smell of an antiseptic (iodoform) appears.
- Let's discuss the mechanism of iodoform reaction. We are taking the reagents as iodine and sodium hydroxide solution along with the substance ($C{{H}_{3}}COR$) which needs to get tested.
- In the first step substitution of all three hydrogens in methyl groups by iodine takes place. It takes place in the presence of hydroxide ions formed from sodium hydroxide. The reaction is given below
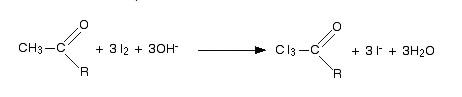
- In the next step, the bond between $C{{I}_{3}}$ and the rest of the molecule is broken. This will lead to the formation of iodoform and salt of an acid. The reaction is shown below
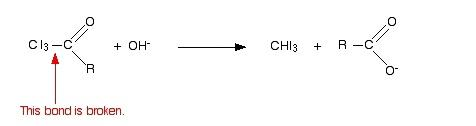
-Hence the overall reaction can be written as follows
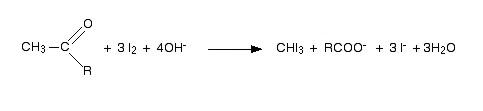
From the above reaction mechanism, it's clear that the presence of methyl ($C{{H}_{3}}$) is mandatory for giving the iodoform test positive. Hence ethanal or acetaldehyde ($C{{H}_{3}}CHO$) is the only compound among options that gives iodoform reaction.
So, the correct answer is “Option A”.
Note: There might be a confusion among the given options. Methyl alcohol ($C{{H}_{3}}OH$) also contains $C{{H}_{3}}$. But it won't give iodoform test because it cannot be oxidized into a compound which contains methyl keto group since methyl alcohol has only one carbon atom.
Complete step by step answer:
- The iodoform test is a test which is used to detect the presence of alcohols with the structure $RCH\left( OH \right)C{{H}_{3}}$ and carbonyl compounds with the structure $RCOC{{H}_{3}}$ . Iodoform is a chemical compound triiodomethane with the chemical formula $CH{{I}_{3}}$ .
- There are two different ways in which we can perform the iodoform reaction. One is by using iodine and sodium hydroxide solution and the other one is by using potassium iodide and sodium hypochlorite solutions.
- If the reaction result is positive or in other words the reactants contain the above-mentioned compounds, then a pale-yellow precipitate with the smell of an antiseptic (iodoform) appears.
- Let's discuss the mechanism of iodoform reaction. We are taking the reagents as iodine and sodium hydroxide solution along with the substance ($C{{H}_{3}}COR$) which needs to get tested.
- In the first step substitution of all three hydrogens in methyl groups by iodine takes place. It takes place in the presence of hydroxide ions formed from sodium hydroxide. The reaction is given below

- In the next step, the bond between $C{{I}_{3}}$ and the rest of the molecule is broken. This will lead to the formation of iodoform and salt of an acid. The reaction is shown below

-Hence the overall reaction can be written as follows

From the above reaction mechanism, it's clear that the presence of methyl ($C{{H}_{3}}$) is mandatory for giving the iodoform test positive. Hence ethanal or acetaldehyde ($C{{H}_{3}}CHO$) is the only compound among options that gives iodoform reaction.
So, the correct answer is “Option A”.
Note: There might be a confusion among the given options. Methyl alcohol ($C{{H}_{3}}OH$) also contains $C{{H}_{3}}$. But it won't give iodoform test because it cannot be oxidized into a compound which contains methyl keto group since methyl alcohol has only one carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




