
Iron carbonyl, \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:
A. Trinuclear
B. Mononuclear
C. Tetranuclear
D. Dinuclear
Answer
612k+ views
Hint: Here, we will proceed by defining the nuclearity of the coordinate compounds. Then draw the structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] to find the nuclearity of the given coordinate compound.
Complete answer:
Definition of Nuclearity: The nuclearity of a single coordination entity indicates the number of central atoms joined by bridging ligands or metal-metal bonds.
The simplest nuclearity is mononuclear, followed by dinuclear, trinuclear, tetranuclear, pentanuclear…………………………., polynuclear.
Iron pentacarbonyl, also known as Iron carbonyl, is the compound with compound with formula \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\].
The structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:
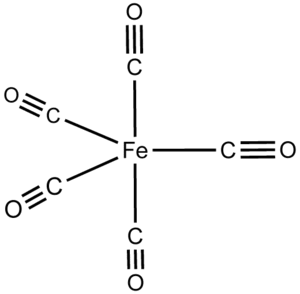
From the structural form of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\], one Fe atom is surrounded by 5 CO ligands.
So, Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is mononuclear.
Note: Under standard conditions \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is a free-flowing, straw-coloured liquid with a pungent smell. Mononuclear complexes are the simplest types of coordination compounds that contain a single metal atom or ion surrounded by monodentate ligands.
Complete answer:
Definition of Nuclearity: The nuclearity of a single coordination entity indicates the number of central atoms joined by bridging ligands or metal-metal bonds.
The simplest nuclearity is mononuclear, followed by dinuclear, trinuclear, tetranuclear, pentanuclear…………………………., polynuclear.
Iron pentacarbonyl, also known as Iron carbonyl, is the compound with compound with formula \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\].
The structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:

From the structural form of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\], one Fe atom is surrounded by 5 CO ligands.
So, Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is mononuclear.
Note: Under standard conditions \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is a free-flowing, straw-coloured liquid with a pungent smell. Mononuclear complexes are the simplest types of coordination compounds that contain a single metal atom or ion surrounded by monodentate ligands.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




