
IUPAC name of tertiary butyl alcohol is:
A. 1-butanol
B. 2-butanol
C. 2-methyl-1-propanol
D.2-methyl-2-propanol
Answer
578.4k+ views
Hint: In tertiary alcohol, the carbon atom is joined with an alcohol group and three other carbon atoms. As the number of carbon in the parent chain increases, the name of hydrocarbon also changes.
Complete step by step answer:
The International Union of pure and applied chemistry has set some guidelines for determining the distinct name for any chemical compound. By following those rules, the IUPAC name of tertiary butyl alcohol is determined as follows.
The structure of the given compound is shown below.
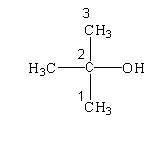
STEP 1: Identify the group
In the given hydrocarbon, an alcohol group is present in the second position. Therefore the suffix will be given as –ol.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of three carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as propane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom is shown in the diagram.
STEP 4: Identify any branched group attached to the carbon
In the second position, one methyl group is present.
STEP 5: Combine the entire element into a single IUPAC name
The IUPAC name of the compound is 2-methyl-2-propanol.
Therefore, the correct option is D.
Note:
There are other names of tertiary butyl alcohol that are tert-butanol or t-butanol. Tertiary butyl alcohol is one of the isomers of butanol. The molecular formula of butanol and tert butyl alcohol is the same but the structures are different.
Complete step by step answer:
The International Union of pure and applied chemistry has set some guidelines for determining the distinct name for any chemical compound. By following those rules, the IUPAC name of tertiary butyl alcohol is determined as follows.
The structure of the given compound is shown below.

STEP 1: Identify the group
In the given hydrocarbon, an alcohol group is present in the second position. Therefore the suffix will be given as –ol.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of three carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as propane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom is shown in the diagram.
STEP 4: Identify any branched group attached to the carbon
In the second position, one methyl group is present.
STEP 5: Combine the entire element into a single IUPAC name
The IUPAC name of the compound is 2-methyl-2-propanol.
Therefore, the correct option is D.
Note:
There are other names of tertiary butyl alcohol that are tert-butanol or t-butanol. Tertiary butyl alcohol is one of the isomers of butanol. The molecular formula of butanol and tert butyl alcohol is the same but the structures are different.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




