
How is the Lewis structure of an ion written?
Answer
552.3k+ views
Hint: To draw the Lewis structure of any molecule or ion, the valence electrons are determined first. While counting the electrons of the compounds carrying charges, the negative ion should contain extra electrons in their Lewis structure and the positive ions should have fewer electrons as compared to the uncharged molecule.
Complete step by step answer:
The Lewis structure is the graphic representation of the valence electrons surrounding the atoms in the molecule. It shows how the electrons are arranged around the atom whether they are present as bonding electrons or as lone pairs of electrons. The valence electrons in the Lewis structure are represented in the form of small dots.
The total number of valence electrons present in the Lewis structure can be determined by counting the valence electrons of the atom present in the molecule and adding the valence electrons of the individual atom.
The valence electrons are defined as the electrons present in the outermost electronic configuration of the chemical element. The valence electrons take part in the bonding process as they can be shared or transferred to other atoms to form molecules.
The Lewis structure of an ion can be drawn the same as we draw for the normal molecule but the difference is only of the charge present in the compound. In case of compound carrying negative charge, the charge of the compound is added to the total valence electrons of the molecule and in case of compound carrying positive charge, the charge of the compound is subtracted from the total valence electrons given by the compound.
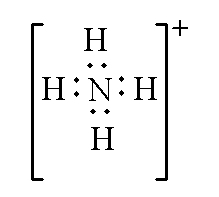
Example: In ammonium ion $NH_4^ +$, the total valence electrons by one nitrogen atom and four hydrogen atoms is 9. As positive charge is present so the valence electrons are subtracted by 1. Therefore, the total valence electron of the ammonium ion will be (9 – 1 =8).
The Lewis structure of ammonium ion is shown below.
In hypochlorite ion $Cl{O^ - }$, the total valence electrons by one chlorine and one oxygen atom is 13 as negative charge is present so 1 is added. Therefore, the total valence electrons of hypochlorite ions is $13 + 1 = 14$.
The Lewis structure of hypochlorite ion is shown below.
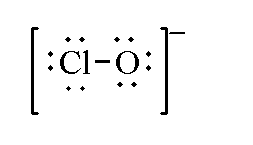
Note: Anion are formed when the electrons are gained therefore the charge of ion it is added to determine the total valence electrons of the ion whereas the cations are formed when loss of electrons take place therefore the charge of ion is subtracted from the total valence electrons.
Complete step by step answer:
The Lewis structure is the graphic representation of the valence electrons surrounding the atoms in the molecule. It shows how the electrons are arranged around the atom whether they are present as bonding electrons or as lone pairs of electrons. The valence electrons in the Lewis structure are represented in the form of small dots.
The total number of valence electrons present in the Lewis structure can be determined by counting the valence electrons of the atom present in the molecule and adding the valence electrons of the individual atom.
The valence electrons are defined as the electrons present in the outermost electronic configuration of the chemical element. The valence electrons take part in the bonding process as they can be shared or transferred to other atoms to form molecules.
The Lewis structure of an ion can be drawn the same as we draw for the normal molecule but the difference is only of the charge present in the compound. In case of compound carrying negative charge, the charge of the compound is added to the total valence electrons of the molecule and in case of compound carrying positive charge, the charge of the compound is subtracted from the total valence electrons given by the compound.
Example: In ammonium ion $NH_4^ +$, the total valence electrons by one nitrogen atom and four hydrogen atoms is 9. As positive charge is present so the valence electrons are subtracted by 1. Therefore, the total valence electron of the ammonium ion will be (9 – 1 =8).
The Lewis structure of ammonium ion is shown below.

In hypochlorite ion $Cl{O^ - }$, the total valence electrons by one chlorine and one oxygen atom is 13 as negative charge is present so 1 is added. Therefore, the total valence electrons of hypochlorite ions is $13 + 1 = 14$.
The Lewis structure of hypochlorite ion is shown below.
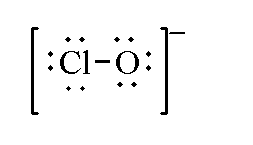
Note: Anion are formed when the electrons are gained therefore the charge of ion it is added to determine the total valence electrons of the ion whereas the cations are formed when loss of electrons take place therefore the charge of ion is subtracted from the total valence electrons.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Who is eligible for RTE class 9 social science CBSE

What is the Full Form of ISI and RAW

How do you find the valency of chlorine sulphur and class 9 chemistry CBSE

What are the major achievements of the UNO class 9 social science CBSE

Explain the importance of pH in everyday life class 9 chemistry CBSE

Differentiate between parenchyma collenchyma and sclerenchyma class 9 biology CBSE





