
Marshall’s acid is:
(A) ${{H}_{2}}{{S}_{2}}{{O}_{5}}$
(B) ${{H}_{2}}{{S}_{2}}{{O}_{8}}$
(C) ${{H}_{2}}S{{O}_{6}}$
(D) ${{H}_{2}}S{{O}_{4}}$
Answer
565.2k+ views
Hint: Marshall’s acid has a peroxide linkage in them.
- It is commonly called as persulphates.
Complete step by step answer:
- In the question four options are given and we have said that among them which one is called Marshall's acid. It is a typical factual type question, in which we have to say the commonly used name of the chemical compounds.
- Now let’s see why Marshall’s acid is called so actually this acid was invented by Professor Hugh Marshall and the acid was given the name after him to show reverence and respect for his invention.
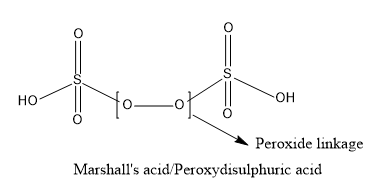
- The Marshall’s acid is an oxy acid of Sulphur and it has a peroxide linkage in them i.e. they have (–O-O-) linkage in them. The Marshall acid is also called as peroxydisulfuric acid.
Now we will find the formulae for the chemical species by decoding the name peroxydisulfuric acid.
- From the name itself we know there is peroxide linkage since there is a peroxy term in the name.
- Then is the term disulphuric acid, it means two moles of sulphuric acid are present and which is connected by peroxide linkage.
- The chemical formula of sulphuric acid is ${{H}_{2}}S{{O}_{4}}$. Two moles of sulphuric acid means it gives a formulae of ${{H}_{4}}{{S}_{2}}{{O}_{8}}$.Since we know that the sulphuric acids are linked by peroxide linkage remove 2 H atoms to get the formula of peroxodisulfuric acid i.e. Marshall’s acid and the formulae is ${{H}_{2}}{{S}_{2}}{{O}_{8}}$.
So, the correct answer is “Option B”.
Note: There is always a chance of getting confused between Caro’s acid and Marshall’s acid. The peroxymonosulfuric acid is called as the Caro’s acid and they have the formulae ${{H}_{2}}{{S}_{2}}{{O}_{5}}$.
- Marshall’s acid is a dibasic acid it can donate two ${{H}^{+}}$ ions during the chemical reaction.
- It is commonly called as persulphates.
Complete step by step answer:
- In the question four options are given and we have said that among them which one is called Marshall's acid. It is a typical factual type question, in which we have to say the commonly used name of the chemical compounds.
- Now let’s see why Marshall’s acid is called so actually this acid was invented by Professor Hugh Marshall and the acid was given the name after him to show reverence and respect for his invention.
- The Marshall’s acid is an oxy acid of Sulphur and it has a peroxide linkage in them i.e. they have (–O-O-) linkage in them. The Marshall acid is also called as peroxydisulfuric acid.
Now we will find the formulae for the chemical species by decoding the name peroxydisulfuric acid.
- From the name itself we know there is peroxide linkage since there is a peroxy term in the name.
- Then is the term disulphuric acid, it means two moles of sulphuric acid are present and which is connected by peroxide linkage.
- The chemical formula of sulphuric acid is ${{H}_{2}}S{{O}_{4}}$. Two moles of sulphuric acid means it gives a formulae of ${{H}_{4}}{{S}_{2}}{{O}_{8}}$.Since we know that the sulphuric acids are linked by peroxide linkage remove 2 H atoms to get the formula of peroxodisulfuric acid i.e. Marshall’s acid and the formulae is ${{H}_{2}}{{S}_{2}}{{O}_{8}}$.

So, the correct answer is “Option B”.
Note: There is always a chance of getting confused between Caro’s acid and Marshall’s acid. The peroxymonosulfuric acid is called as the Caro’s acid and they have the formulae ${{H}_{2}}{{S}_{2}}{{O}_{5}}$.
- Marshall’s acid is a dibasic acid it can donate two ${{H}^{+}}$ ions during the chemical reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




