
What is meant by hemiacetal? Give an example of a reaction (with mechanism) showing its formation.
Answer
585.6k+ views
Hint: A hemiacetal which is taken from a Greek word ‘hemi’ which means half, is formed when an aldehyde reacts with an alcohol. This can be done either through a neutral reaction or through an acid catalysed reaction.
Complete step by step answer:
A hemiacetal is a carbon compound where the central carbon atom is connected to four different molecules, compound or atoms. One of them is an alcohol $\left( { - OH} \right)$ group, the other is an ether $\left( { - OR} \right)$ group. Among rest two, one is an alkyl group denoted by ${\text{R}}$, and other is a hydrogen atom $\left( { - H} \right)$. Here, the carbon chain ${\text{R}}$ can be of any length.
The mechanism of formation of a hemiacetal:
Step (1): Through the neutral reaction mechanism: It involves only the aldehyde, and the alcohol. The steps are shown in the figure below.
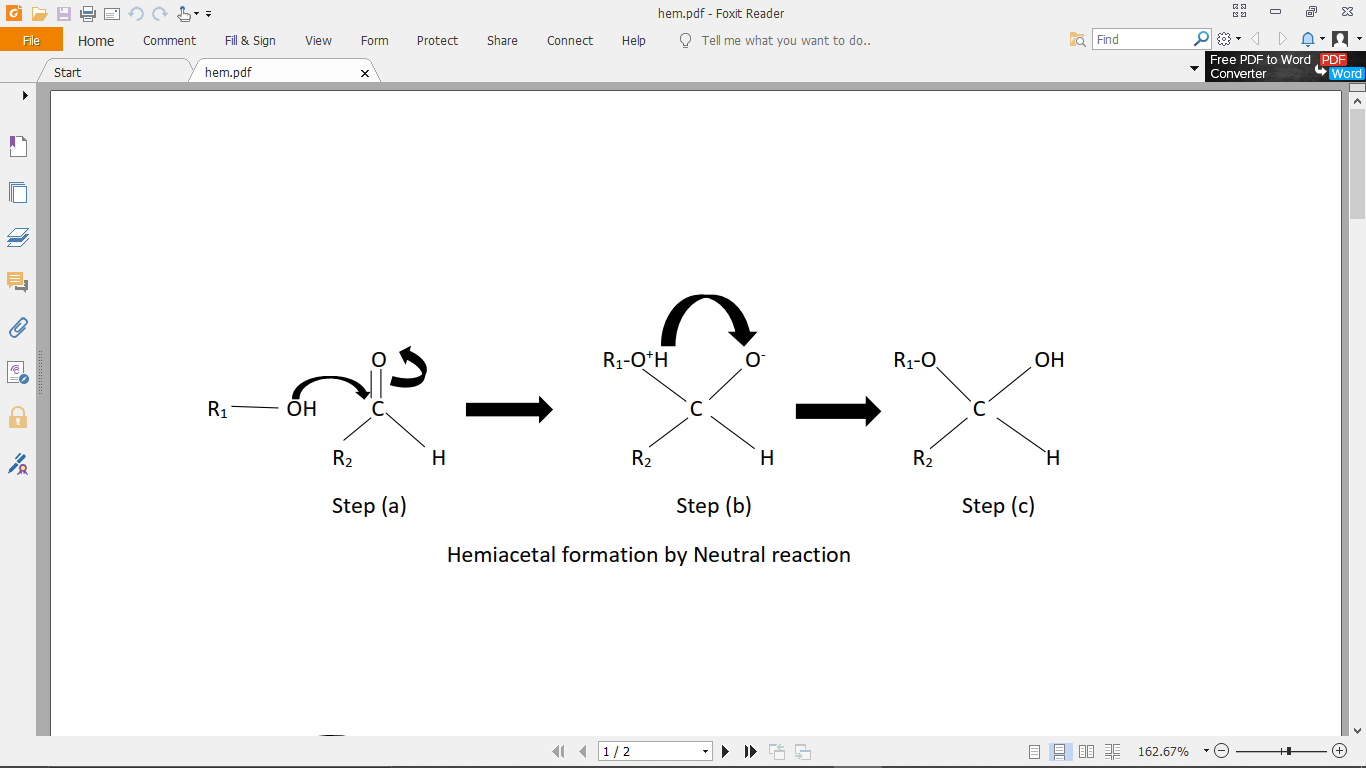
(a) First, the alcohol attacks the carbonyl carbon of the aldehyde.
(b) The pi-electrons from the double bond between the carbon, and oxygen go to the oxygen atom, making oxygen atom negatively charged, and carbon atom positively charged.
(c) The extra proton on the alcohol group which got attached is then transferred to the negatively charged oxygen, and forms the hemiacetal.
Step (2): Through the acid catalysed mechanism: It involves the catalyzation of aldehyde first. The steps are shown in the figure below.
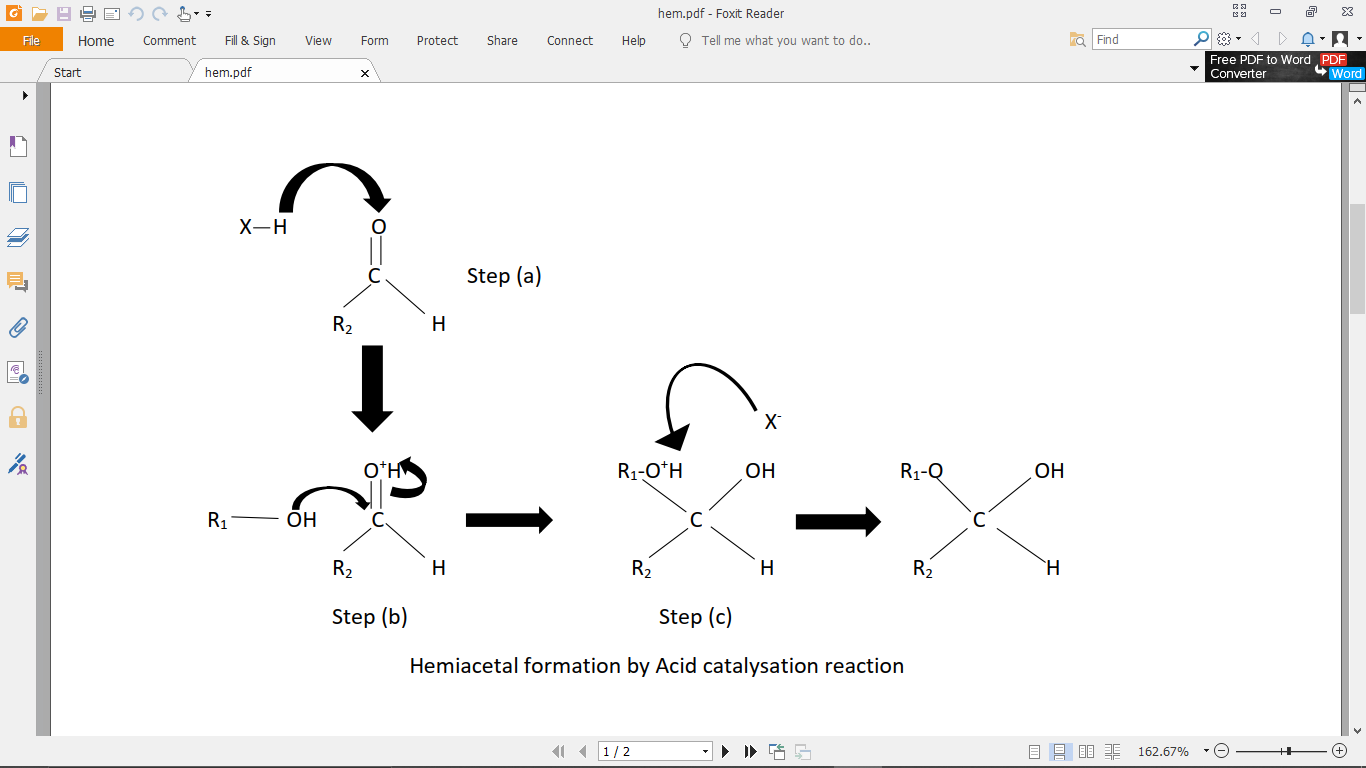
(a) First, the acidic hydrogen from the acid $\left( {X - H} \right)$ attacks the lone pair of oxygen in the aldehyde. This puts a positive charge on the oxygen atom of the aldehyde.
(b) The positive charge on the electronegative atom, oxygen is highly unstable, so, the pi-bond between the oxygen, and carbon of the aldehyde breaks, and the electron neutralises the positive of the oxygen atom. This creates a positive charge on the carbonyl carbon as shown in figure (b). Now, the alcohol being nucleophilic in nature, attacks the positive carbon centre.
(c) The extra proton on the alcohol is then taken by the conjugate base of the acid used in step (a), that is $\left( {X - H} \right)$. Hence, hemiacetal is formed.
Note:
For effective formation, acid catalyzation gives faster results because without it, the extra electron of the oxygen after the alcohol attack, can go back to form the double bond giving back the initial product. That’s why neutral reaction is a slow step reaction.
Complete step by step answer:
A hemiacetal is a carbon compound where the central carbon atom is connected to four different molecules, compound or atoms. One of them is an alcohol $\left( { - OH} \right)$ group, the other is an ether $\left( { - OR} \right)$ group. Among rest two, one is an alkyl group denoted by ${\text{R}}$, and other is a hydrogen atom $\left( { - H} \right)$. Here, the carbon chain ${\text{R}}$ can be of any length.
The mechanism of formation of a hemiacetal:
Step (1): Through the neutral reaction mechanism: It involves only the aldehyde, and the alcohol. The steps are shown in the figure below.

(a) First, the alcohol attacks the carbonyl carbon of the aldehyde.
(b) The pi-electrons from the double bond between the carbon, and oxygen go to the oxygen atom, making oxygen atom negatively charged, and carbon atom positively charged.
(c) The extra proton on the alcohol group which got attached is then transferred to the negatively charged oxygen, and forms the hemiacetal.
Step (2): Through the acid catalysed mechanism: It involves the catalyzation of aldehyde first. The steps are shown in the figure below.

(a) First, the acidic hydrogen from the acid $\left( {X - H} \right)$ attacks the lone pair of oxygen in the aldehyde. This puts a positive charge on the oxygen atom of the aldehyde.
(b) The positive charge on the electronegative atom, oxygen is highly unstable, so, the pi-bond between the oxygen, and carbon of the aldehyde breaks, and the electron neutralises the positive of the oxygen atom. This creates a positive charge on the carbonyl carbon as shown in figure (b). Now, the alcohol being nucleophilic in nature, attacks the positive carbon centre.
(c) The extra proton on the alcohol is then taken by the conjugate base of the acid used in step (a), that is $\left( {X - H} \right)$. Hence, hemiacetal is formed.
Note:
For effective formation, acid catalyzation gives faster results because without it, the extra electron of the oxygen after the alcohol attack, can go back to form the double bond giving back the initial product. That’s why neutral reaction is a slow step reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




