
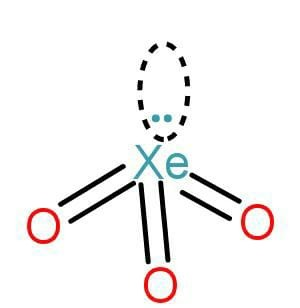
Mention the shape and draw a diagram of \[{\text{Xe}}{{\text{O}}_3}\] .
Answer
563.1k+ views
Hint:The hybridisation of \[{\text{Xe}}{{\text{O}}_3}\] is \[{\text{s}}{{\text{p}}^3}\] . The geometry and shape in case of the above molecule will be different due to the presence of the one lone pair.
Complete answer:
To find out the shape and geometry we first need to calculate the hybridisation. Xenon is a group number 18 element or is a Noble gas. They have 8 electrons in its valence shell. Oxygen is a bivalent species. It belongs to group number 16 and has 6 electrons in its valence shell. It needs just 2 electrons to complete its octet and hence it attaches via a double bond. The formula for calculation of hybridisation is:
\[{\text{Steric number}} = \dfrac{1}{2}\left[ {{\text{V }} + {\text{M}}{\text{.S}} + {\text{A}} - {\text{C}}} \right]\]
V is number of valence electrons on central atom, m.s is no of species attached via single bond or number of monovalent species, A is charge on anion and C is cationic charge,
In the given molecule that is \[{\text{Xe}}{{\text{O}}_3}\] the central element is xenon and hence, the number of valence electrons is 8. Oxygen is a bivalent species and hence the number of monovalent species is zero. The molecule is neutral and hence there is no cationic or anionic charge.
The steric number will be \[{\text{Steric number}} = \dfrac{1}{2}\left[ 8 \right] = 4\]
The geometry with the steric number 4 is tetrahedral, that is \[{\text{s}}{{\text{p}}^3}\] . As there are 3 oxygen attached there is one lone pair present. The lone pairs are not included in the shape of the molecule. Hence the shape will be pyramidal.
Note:
Xenon has a complete octet so technically xenon does not need to form any bond because it is already very stable. But in the presence of more electronegative elements such as fluorine and oxygen it is found to be forming bonds as they can bring orbital excitations.
Complete answer:
To find out the shape and geometry we first need to calculate the hybridisation. Xenon is a group number 18 element or is a Noble gas. They have 8 electrons in its valence shell. Oxygen is a bivalent species. It belongs to group number 16 and has 6 electrons in its valence shell. It needs just 2 electrons to complete its octet and hence it attaches via a double bond. The formula for calculation of hybridisation is:
\[{\text{Steric number}} = \dfrac{1}{2}\left[ {{\text{V }} + {\text{M}}{\text{.S}} + {\text{A}} - {\text{C}}} \right]\]
V is number of valence electrons on central atom, m.s is no of species attached via single bond or number of monovalent species, A is charge on anion and C is cationic charge,
In the given molecule that is \[{\text{Xe}}{{\text{O}}_3}\] the central element is xenon and hence, the number of valence electrons is 8. Oxygen is a bivalent species and hence the number of monovalent species is zero. The molecule is neutral and hence there is no cationic or anionic charge.
The steric number will be \[{\text{Steric number}} = \dfrac{1}{2}\left[ 8 \right] = 4\]
The geometry with the steric number 4 is tetrahedral, that is \[{\text{s}}{{\text{p}}^3}\] . As there are 3 oxygen attached there is one lone pair present. The lone pairs are not included in the shape of the molecule. Hence the shape will be pyramidal.

Note:
Xenon has a complete octet so technically xenon does not need to form any bond because it is already very stable. But in the presence of more electronegative elements such as fluorine and oxygen it is found to be forming bonds as they can bring orbital excitations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




