
Meso tartaric acid is optically inactive due to the presence of:
A. Molecular symmetry
B. Molecular asymmetry
C. External compensation
D. Two asymmetric C-atoms
Answer
590.4k+ views
Hint: Symmetrical molecules which have two or more than two chiral carbons is known as meso form. Chiral carbon is a carbon bonded with four different types of groups.
Complete step by step answer:
Meso tartaric acid has a plane of symmetry and is achiral.
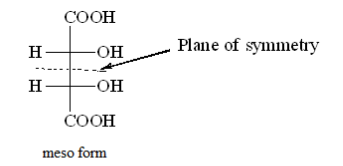
Meso tartaric acid is optically inactive because of internal compensation that means one half of the molecule is neutralized by another.
Hence, the correct option is A, molecular symmetry.
Additional Information:
Tartaric acid naturally occurs in fruits like grapes, bananas, citrus and tamarinds. Its salt, potassium bitartrate, properly known as tartar cream, naturally accumulates during the fermentation process.
As we know that elements of symmetry decide a molecule is chiral or achiral. If a molecule has no plane of symmetry, no centre of symmetry and no alternating axis of symmetry and non superimposable mirror image then the molecule is optically active.
Plane of symmetry breaks a molecule into two equal parts which are related as objects and mirror images.
A centre of symmetry in a molecule is said to exist if a line is drawn from any atom to the central point and when extended to equal distance beyond this point, meets the identical atom or group. It is usually present in even numbered ring
Note:
the appearance of symmetric molecules does not change. Dissymmetric molecules containing at least one asymmetric carbon are called asymmetric molecules.
Complete step by step answer:
Meso tartaric acid has a plane of symmetry and is achiral.

Meso tartaric acid is optically inactive because of internal compensation that means one half of the molecule is neutralized by another.
Hence, the correct option is A, molecular symmetry.
Additional Information:
Tartaric acid naturally occurs in fruits like grapes, bananas, citrus and tamarinds. Its salt, potassium bitartrate, properly known as tartar cream, naturally accumulates during the fermentation process.
As we know that elements of symmetry decide a molecule is chiral or achiral. If a molecule has no plane of symmetry, no centre of symmetry and no alternating axis of symmetry and non superimposable mirror image then the molecule is optically active.
Plane of symmetry breaks a molecule into two equal parts which are related as objects and mirror images.
A centre of symmetry in a molecule is said to exist if a line is drawn from any atom to the central point and when extended to equal distance beyond this point, meets the identical atom or group. It is usually present in even numbered ring
Note:
the appearance of symmetric molecules does not change. Dissymmetric molecules containing at least one asymmetric carbon are called asymmetric molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




