
Name the reagent used in the following reactions: Bromination of phenol to $ 2,4,6\text{ } $ tribromophenol.
Answer
529.2k+ views
Hint :We know that the bromination is a type of halogenation reaction. The halogenation is defined as the type of the reaction which involves the replacement of the atom of the halogen with the other substance where we see the atom of halogen ends up as the part of that compound. But during the bromination of phenol into $ 2,4,6 $ tribromo phenol the reagent is used which helps in substituting the three atoms of the bromine in the molecule of phenol.
Complete Step By Step Answer:
So in the bromination of phenol the solvent used has a greater influence in processing the reaction. so in different solvents the formation of different products takes place. So to convert the bromine into $ 2,4,6 $ tribromophenol the reagent used is bromine water, in the water the process of ionisation is facilitated. The phenol tends to get ionized to form the phenoxide ion which is considered as a better ortho –para directing. The bromine also tends to get ionised into the large extent of the bromine ions. And the stability of the bromine ions is more even in water or ionic solvents. So this tends to form the strong ortho para directing group and the bromine ion gets more stabilised and forms the tribromophenol. So the reaction of the bromination of phenol by bromine water is the following:
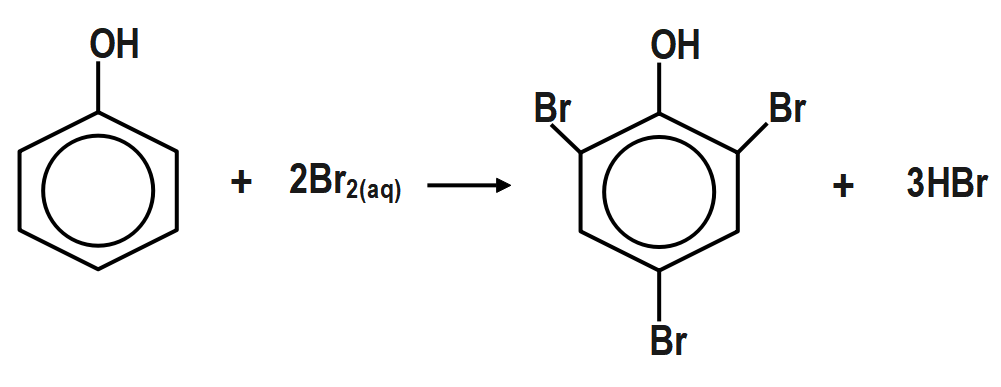
So the formation of $ 2,4,6 $ tribromophenol takes place with the help of the reagent called bromine water.
Note :
The formation of ortho bromo phenol and para bromo phenol takes place when the bromine in carbon disulphide is reacted with phenol. The para bromophenol dominates in both of the product forms. The para and ortho bromophenol formation takes place because the carbon disulphide is not ionised in the water so the bromine ions are not ionised to large extent in water.
Complete Step By Step Answer:
So in the bromination of phenol the solvent used has a greater influence in processing the reaction. so in different solvents the formation of different products takes place. So to convert the bromine into $ 2,4,6 $ tribromophenol the reagent used is bromine water, in the water the process of ionisation is facilitated. The phenol tends to get ionized to form the phenoxide ion which is considered as a better ortho –para directing. The bromine also tends to get ionised into the large extent of the bromine ions. And the stability of the bromine ions is more even in water or ionic solvents. So this tends to form the strong ortho para directing group and the bromine ion gets more stabilised and forms the tribromophenol. So the reaction of the bromination of phenol by bromine water is the following:

So the formation of $ 2,4,6 $ tribromophenol takes place with the help of the reagent called bromine water.
Note :
The formation of ortho bromo phenol and para bromo phenol takes place when the bromine in carbon disulphide is reacted with phenol. The para bromophenol dominates in both of the product forms. The para and ortho bromophenol formation takes place because the carbon disulphide is not ionised in the water so the bromine ions are not ionised to large extent in water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




