
One cyclic acetal form of D-galactose is shown.
Which name most completely describes this cyclic acetal form?
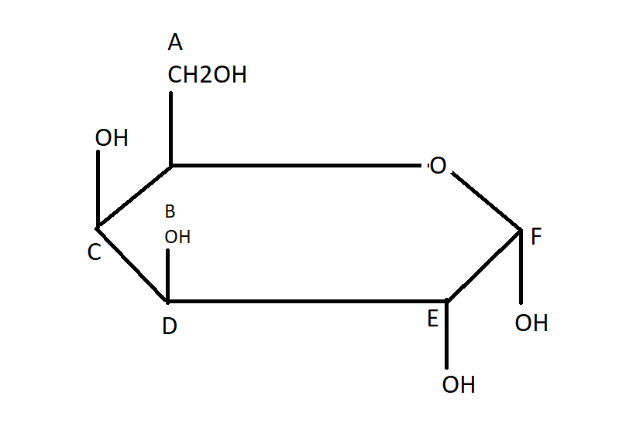
$A.\alpha - D - Galactofuranose$
$B.\beta - P - Galactofuranose$
$C.\alpha - D - Galactopyranose$
$D.\beta - P - Galactopyranose$

Answer
575.4k+ views
Hint: Here, the carbon atom refers to the very first carbon atom, which attaches to a functional group. Also, the hydroxyl group on carbon-5 is on the right side of the Fischer projection. It is a six-membered ring.
Complete answer:
Full name consists of anomeric carbon, i.e., whether it’s $\alpha $, or $\beta $. So, alpha $\left( \alpha \right)$ carbon in an organic compound refers to the very first carbon atom, which attaches to a functional group.
Then, the configuration of the last chiral center, i.e., whether it is D or L. So, in the case of a galactose when the hydroxyl group on carbon-5 is on the right side of the Fischer projection, the galactose is of D- configuration.
Then we should lastly put the name of carbohydrate with the name of the cyclic form.
As it is a six-membered ring in the given figure, the name is $\alpha - D - Galactopyranose$.
Now, what the anomeric carbon is. Simply, it is the carbon, which is derived from the carbonyl carbon compound (the ketone or aldehyde functional group). This carbonyl carbon compound must be in its open-chain form of the carbohydrate molecule and the anomerization is the process of conversion of one anomer (cyclic monosaccharides or glycosides that are epimers) to the other.
Now, a chiral center is an atom, which has four different groups attached to it in such a pattern that it must have a non-superimposable mirror image. So, if we draw the mirror image of any molecule and see that they are different, then the molecule is chiral and if they are the same, then it is not chiral.
Hence the correct answer is option C.
Note:
It is to be noted that the cyclic acetals are five or higher-membered monomers with at least one unit in which two oxygen atoms take part on the sides by substituted or unsubstituted methylene group. This is formed by the reaction of two molecules, a ketone and a diol.
Complete answer:
Full name consists of anomeric carbon, i.e., whether it’s $\alpha $, or $\beta $. So, alpha $\left( \alpha \right)$ carbon in an organic compound refers to the very first carbon atom, which attaches to a functional group.
Then, the configuration of the last chiral center, i.e., whether it is D or L. So, in the case of a galactose when the hydroxyl group on carbon-5 is on the right side of the Fischer projection, the galactose is of D- configuration.
Then we should lastly put the name of carbohydrate with the name of the cyclic form.
As it is a six-membered ring in the given figure, the name is $\alpha - D - Galactopyranose$.
Now, what the anomeric carbon is. Simply, it is the carbon, which is derived from the carbonyl carbon compound (the ketone or aldehyde functional group). This carbonyl carbon compound must be in its open-chain form of the carbohydrate molecule and the anomerization is the process of conversion of one anomer (cyclic monosaccharides or glycosides that are epimers) to the other.
Now, a chiral center is an atom, which has four different groups attached to it in such a pattern that it must have a non-superimposable mirror image. So, if we draw the mirror image of any molecule and see that they are different, then the molecule is chiral and if they are the same, then it is not chiral.
Hence the correct answer is option C.
Note:
It is to be noted that the cyclic acetals are five or higher-membered monomers with at least one unit in which two oxygen atoms take part on the sides by substituted or unsubstituted methylene group. This is formed by the reaction of two molecules, a ketone and a diol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




