
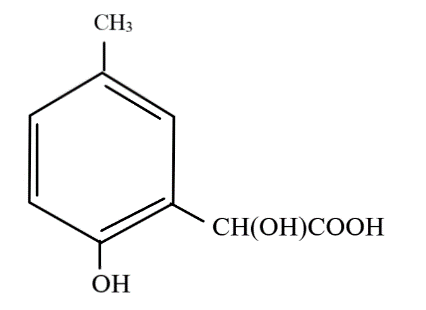
p-Cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form the compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is?
A.
B.
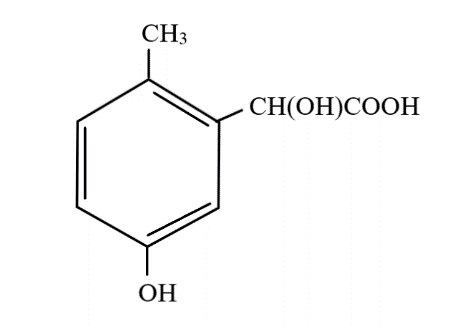
C.
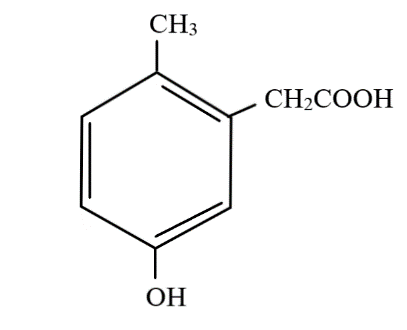
D.
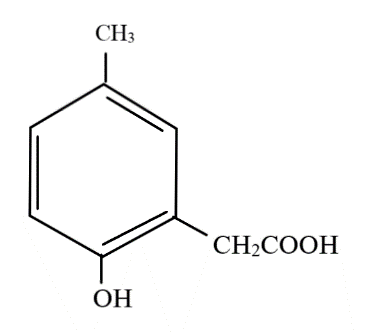




Answer
573.6k+ views
Hint: The reaction of chloroform in an alkaline medium with phenol derivatives like cresol is an example of the Riemer-Tiemann reaction, in which an aldehyde group is introduced at the ortho position. Addition of hydrogen cyanide leads to the formation of cyanohydrin at this site, which will hydrolyse to give the corresponding acid.
Complete step by step answer:
The first step in the reaction is the popular Reimer-Tiemann reaction, in which phenol and its derivatives react with chloroform in alkaline medium to give an aldehyde at the ortho position to the phenolic group, otherwise known as an ortho hydroxy benzaldehyde. Thus, a $ - CHO$ group is introduced at the ortho position to the $ - OH$ group to form compound A, which has the formula $p - C{H_3}(o - CHO){C_6}{H_3}OH$
Addition of hydrogen cyanide forms a cyanohydrin at the site of the carbonyl carbon and due to the attack of the nucleophile ($C{N^ - }$ ion) the double bond between carbon and oxygen breaks and the electron pair goes to oxygen, which accepts the hydrogen ion from the hydrogen cyanide to form an alcohol, to form compound B, which has the formula $p - C{H_3}(o - CH(CN)(OH)){C_6}{H_3}OH$
Upon acidic hydrolysis, the cyanide group gets oxidised and forms a carboxylic acid. As the carbon atom directly attached to the benzene ring now has four different molecules bonded to it, it is known as a chiral carbon.
The full process is shown in the image below. The chiral carbon is marked with the red dot.
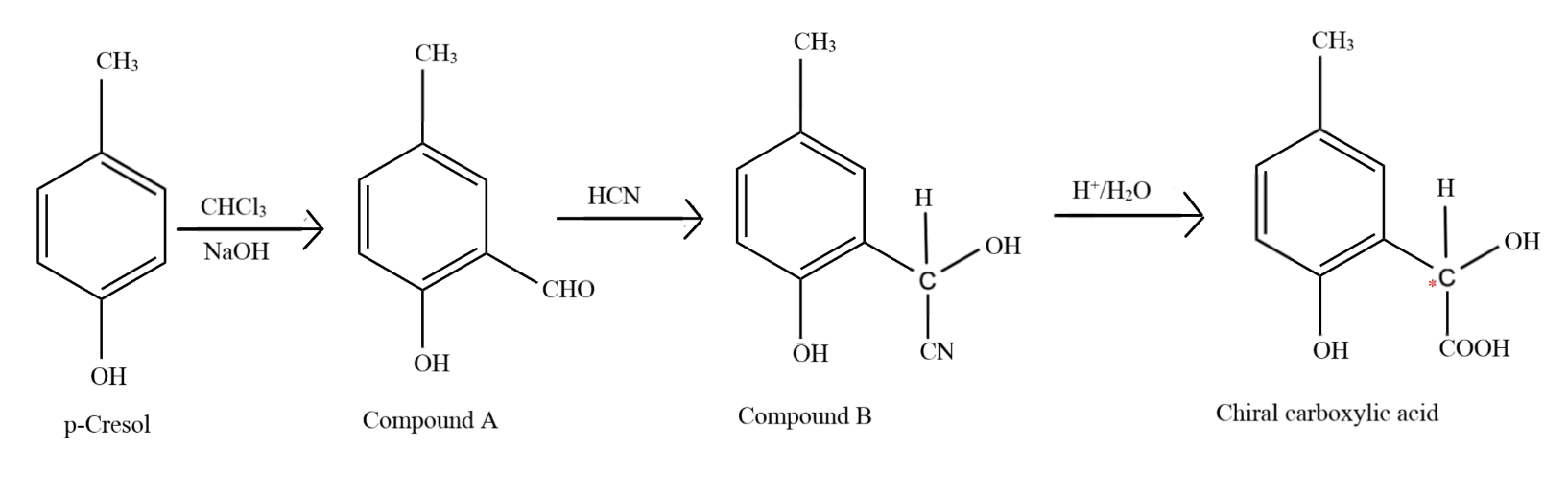
So, the correct answer is Option A.
Note: The reaction intermediate in the Reimer-Tiemann reaction is a highly unstable molecule known as dichlorocarbene, which acts as the initial electrophile. The $ - CHO$ group gets attached only at the ortho position due to the ortho para directive influence of the phenolic group. Note that as the final compound is a chiral carboxylic acid, it will show optical activity.
Complete step by step answer:
The first step in the reaction is the popular Reimer-Tiemann reaction, in which phenol and its derivatives react with chloroform in alkaline medium to give an aldehyde at the ortho position to the phenolic group, otherwise known as an ortho hydroxy benzaldehyde. Thus, a $ - CHO$ group is introduced at the ortho position to the $ - OH$ group to form compound A, which has the formula $p - C{H_3}(o - CHO){C_6}{H_3}OH$
Addition of hydrogen cyanide forms a cyanohydrin at the site of the carbonyl carbon and due to the attack of the nucleophile ($C{N^ - }$ ion) the double bond between carbon and oxygen breaks and the electron pair goes to oxygen, which accepts the hydrogen ion from the hydrogen cyanide to form an alcohol, to form compound B, which has the formula $p - C{H_3}(o - CH(CN)(OH)){C_6}{H_3}OH$
Upon acidic hydrolysis, the cyanide group gets oxidised and forms a carboxylic acid. As the carbon atom directly attached to the benzene ring now has four different molecules bonded to it, it is known as a chiral carbon.
The full process is shown in the image below. The chiral carbon is marked with the red dot.

So, the correct answer is Option A.
Note: The reaction intermediate in the Reimer-Tiemann reaction is a highly unstable molecule known as dichlorocarbene, which acts as the initial electrophile. The $ - CHO$ group gets attached only at the ortho position due to the ortho para directive influence of the phenolic group. Note that as the final compound is a chiral carboxylic acid, it will show optical activity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




