
Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give:
(A) Phenolphthalein
(B) alizarin
(C) coumarin
(D) fluorescein
Answer
573.9k+ views
Hint: Phthalic acid is a dicarboxylic acid. It reacts with a dehydrating agent i.e. sulphuric acid. Phthalic acid loses water on heating and acts as a dehydrating agent. The reaction of phthalic anhydride with resorcinol results in the formation of organic dye. This dye has a wide application as a fluorescent material in the laser application.
Complete Solution :
We have been provided that phthalic acid with resorcinol in the presence of concentrated sulphuric acid,
Phthalic acid is an aromatic dicarboxylic acid, with formula ${{C}_{6}}{{H}_{4}}{{(C{{O}_{2}}H)}_{2}}$ .It is an isomer of isophthalic acid and terephthalic acid. Resorcinol is an organic compound with the formula ${{C}_{6}}{{H}_{4}}{{(OH)}_{2}}$. It is one of three isomeric benzenediols.
- When these two compounds react together in presence of concentrated sulphuric acid,
Sulphuric acid acts as a dehydrating agent because a substance that absorbs moisture from its surroundings is called a dehydrating agent. Sulphuric acid readily protonated ${{H}_{2}}O$ leading to the formation of hydronium ions.
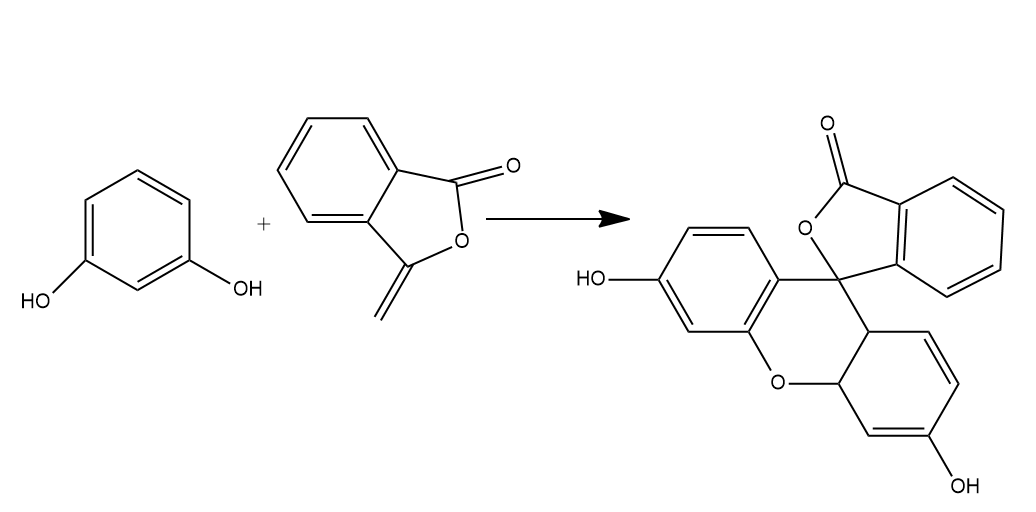
- Phthalic acid reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein. Phthalic acid gets dehydrated on treatment with concentrated ${{H}_{2}}S{{O}_{4}}$ to form phthalic anhydride. Phthalic anhydride further reacts with resorcinol to form fluorescein.
- So, we can say that Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein.
So, the correct answer is “Option D”.
Note: Note that Adolf Von Baeyer first synthesized fluorescein in the laboratory. He prepared it from the phallic anhydride and resorcinol in presence of zinc chloride through the Friedel-Crafts reaction. There is one more method for synthesis using methane sulphonic acid . This gives a high yield.
Sulphuric acid is not used as a drying agent ${{H}_{2}}S$ because it reacts with it to form sulphur.
$\text{ }{{H}_{2}}S{{O}_{4}}+{{H}_{2}}S\to 2{{H}_{2}}O+S{{O}_{2}}+S\text{ }$
Complete Solution :
We have been provided that phthalic acid with resorcinol in the presence of concentrated sulphuric acid,
Phthalic acid is an aromatic dicarboxylic acid, with formula ${{C}_{6}}{{H}_{4}}{{(C{{O}_{2}}H)}_{2}}$ .It is an isomer of isophthalic acid and terephthalic acid. Resorcinol is an organic compound with the formula ${{C}_{6}}{{H}_{4}}{{(OH)}_{2}}$. It is one of three isomeric benzenediols.
- When these two compounds react together in presence of concentrated sulphuric acid,
Sulphuric acid acts as a dehydrating agent because a substance that absorbs moisture from its surroundings is called a dehydrating agent. Sulphuric acid readily protonated ${{H}_{2}}O$ leading to the formation of hydronium ions.

- Phthalic acid reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein. Phthalic acid gets dehydrated on treatment with concentrated ${{H}_{2}}S{{O}_{4}}$ to form phthalic anhydride. Phthalic anhydride further reacts with resorcinol to form fluorescein.
- So, we can say that Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein.
So, the correct answer is “Option D”.
Note: Note that Adolf Von Baeyer first synthesized fluorescein in the laboratory. He prepared it from the phallic anhydride and resorcinol in presence of zinc chloride through the Friedel-Crafts reaction. There is one more method for synthesis using methane sulphonic acid . This gives a high yield.
Sulphuric acid is not used as a drying agent ${{H}_{2}}S$ because it reacts with it to form sulphur.
$\text{ }{{H}_{2}}S{{O}_{4}}+{{H}_{2}}S\to 2{{H}_{2}}O+S{{O}_{2}}+S\text{ }$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




