
What the process of a liquid changing into vapour is called?
Answer
588.9k+ views
Hint: The liquid can be converted into the vapour state by applying the heat. Heat increases the kinetic energy of the particles and thus reduces the force of attraction between the particles. The particles get separated and escape to the vapour state. This process is called vaporization.
Complete step by step solution:
The vaporization is defined as the process by which the liquid state of a substance changes to the vapour state. The kinetic energy (energy possessed by the moving particles) increases with the increase in the temperature of the liquid. Due to increases in the kinetic energy of the molecule, the force of attraction between the molecules decreases. Because of this, the molecules can easily escape from the liquid state into the surrounding in the form of vapours.
Thus the process of changing a liquid into the vapours is called the vaporization. The process involves the absorption of heat energy.
The process can be depicted as follows. The liquid is filled in the beaker is subjected to the heat energy. As heat energy increases, the molecules experience the zero or less force of attraction. They easily escape from the liquid state to the gaseous state.
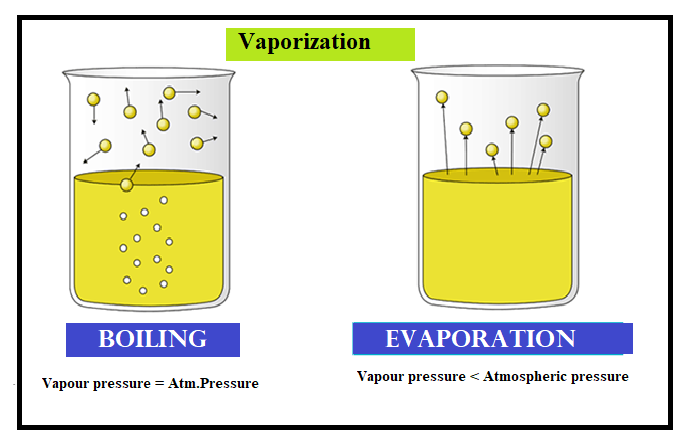
-The vaporization process can be divided into two types.
1)Evaporation
2) Boiling
The boiling point is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure.
There are different factors which affect the rate of vaporization. Some are listed below:
a) Temperature: The rate of vaporization of a liquid is directly related to the temperature. As the temperature of the liquid increases the molecules experience higher kinetic energy, as a result, the force of attraction decreases. Thus the rate of evaporation increases.
b) Surface area: The vaporization of the substance increases with the increase in the surface area of the substance as the more number of particles are exposed to the heat.
c) Pressure: Evaporation is inversely related to the pressure.as the pressure increases it gets difficult for the particles to gain the kinetic energy and escape.
Some of the examples of vaporization are as follows:
1) Salt is obtained from the sea-water by the process of vaporization.
2) Vaporization process is used to dry the wet clothes.
3) The vaporization process is used in industries for the separation of components in the mixture.
Note: The boiling point is different from the evaporation of the substance. The boiling point is a bulk phenomenon and evaporation is a surface phenomenon. Thus one should not consider both as the same.
Complete step by step solution:
The vaporization is defined as the process by which the liquid state of a substance changes to the vapour state. The kinetic energy (energy possessed by the moving particles) increases with the increase in the temperature of the liquid. Due to increases in the kinetic energy of the molecule, the force of attraction between the molecules decreases. Because of this, the molecules can easily escape from the liquid state into the surrounding in the form of vapours.
Thus the process of changing a liquid into the vapours is called the vaporization. The process involves the absorption of heat energy.
The process can be depicted as follows. The liquid is filled in the beaker is subjected to the heat energy. As heat energy increases, the molecules experience the zero or less force of attraction. They easily escape from the liquid state to the gaseous state.

-The vaporization process can be divided into two types.
1)Evaporation
2) Boiling
The boiling point is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure.
There are different factors which affect the rate of vaporization. Some are listed below:
a) Temperature: The rate of vaporization of a liquid is directly related to the temperature. As the temperature of the liquid increases the molecules experience higher kinetic energy, as a result, the force of attraction decreases. Thus the rate of evaporation increases.
b) Surface area: The vaporization of the substance increases with the increase in the surface area of the substance as the more number of particles are exposed to the heat.
c) Pressure: Evaporation is inversely related to the pressure.as the pressure increases it gets difficult for the particles to gain the kinetic energy and escape.
Some of the examples of vaporization are as follows:
1) Salt is obtained from the sea-water by the process of vaporization.
2) Vaporization process is used to dry the wet clothes.
3) The vaporization process is used in industries for the separation of components in the mixture.
Note: The boiling point is different from the evaporation of the substance. The boiling point is a bulk phenomenon and evaporation is a surface phenomenon. Thus one should not consider both as the same.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




