
What Is Rutherford’s atomic model? What are the reasons for failure?
Answer
503.1k+ views
Hint: Rutherford Atomic Model - J. J. Thomson's plum pudding model failed to explain some experimental results related to atomic structure of elements. Ernest Rutherford, a British physicist, carried out an experiment and, based on the results, suggested the atomic structure of elements, coining the term "Rutherford Atomic Model."
Complete answer:
The Alpha Scattering Experiment of Rutherford:
Rutherford carried out an experiment in which he bombarded a thin sheet of gold foil with -particles and then analysed the track of these particles after they collided with the gold foil.
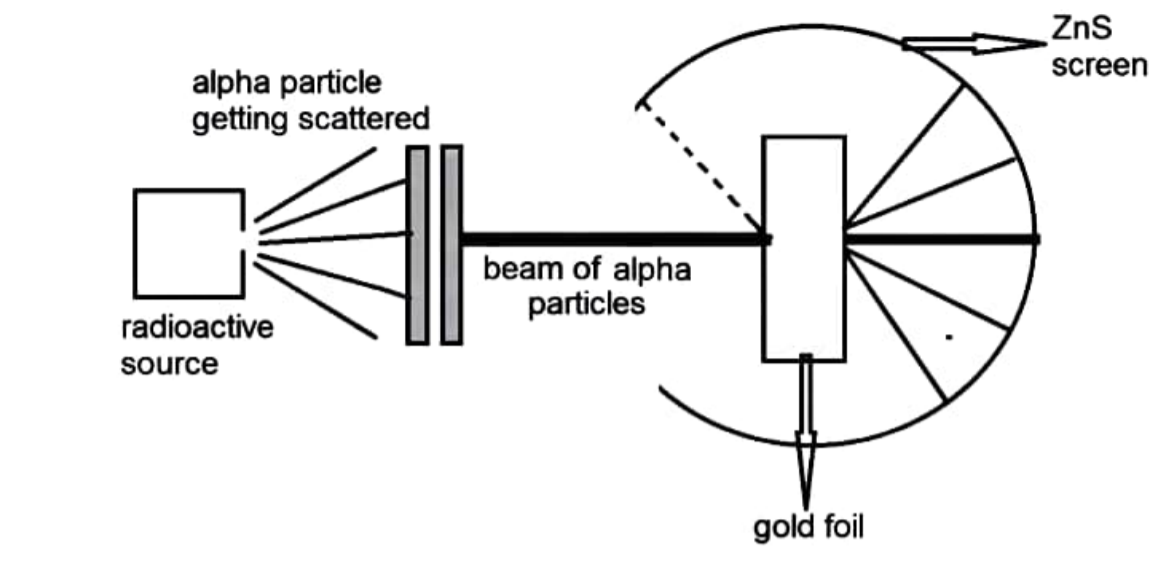
In his experiment, Rutherford focused high-energy -particle streams from a radioactive source at a thin sheet of gold (100 nm thickness). He wrapped a fluorescent zinc sulphide screen around the thin gold foil to investigate the deflection generated by the -particles. Certain observations obtained by Rutherford contradicted Thomson's atomic model.
Atomic Model of Rutherford
Rutherford suggested the atomic structure of elements based on the preceding findings and conclusions. The Rutherford atomic model states:
Positively charged particles and the majority of an atom's mass were concentrated in a very small volume. This part of the atom was dubbed a nucleus by him.
The Rutherford model stated that the nucleus of an atom is surrounded by negatively charged electrons. He also claimed that the electrons that surround the nucleus travel in circular routes at great speeds. These circular routes were given the name orbits by him.
The nucleus is held together by a strong electrostatic force of attraction because electrons are negatively charged and the nucleus is a densely concentrated mass of positively charged particles.
Rutherford's atomic model failed for the reasons listed below.
(1) It was unable to explain the electrons' orbital stability.
2)Electrons revolving in s. orbits accelerate charged particles, which release energy-carrying electromagnetic radiation.
3)The electron will spiral along a spiral pattern as it loses energy, eventually falling into the nucleus. Electrons cannot exist outside the atom in this fashion.
Note:
As an explanation for the surprising experimental results, Rutherford proposed his own physical model for subatomic structure. The atom is made up of a core charge (the current atomic nucleus), which is surrounded by a cloud of (supposedly) orbiting electrons. Rutherford did not use the term "nucleus" in his work. Rutherford only committed himself to a small centre region of very high positive or negative charge in the atom in this May \[1911\] publication.
Complete answer:
The Alpha Scattering Experiment of Rutherford:
Rutherford carried out an experiment in which he bombarded a thin sheet of gold foil with -particles and then analysed the track of these particles after they collided with the gold foil.

In his experiment, Rutherford focused high-energy -particle streams from a radioactive source at a thin sheet of gold (100 nm thickness). He wrapped a fluorescent zinc sulphide screen around the thin gold foil to investigate the deflection generated by the -particles. Certain observations obtained by Rutherford contradicted Thomson's atomic model.
Atomic Model of Rutherford
Rutherford suggested the atomic structure of elements based on the preceding findings and conclusions. The Rutherford atomic model states:
Positively charged particles and the majority of an atom's mass were concentrated in a very small volume. This part of the atom was dubbed a nucleus by him.
The Rutherford model stated that the nucleus of an atom is surrounded by negatively charged electrons. He also claimed that the electrons that surround the nucleus travel in circular routes at great speeds. These circular routes were given the name orbits by him.
The nucleus is held together by a strong electrostatic force of attraction because electrons are negatively charged and the nucleus is a densely concentrated mass of positively charged particles.
Rutherford's atomic model failed for the reasons listed below.
(1) It was unable to explain the electrons' orbital stability.
2)Electrons revolving in s. orbits accelerate charged particles, which release energy-carrying electromagnetic radiation.
3)The electron will spiral along a spiral pattern as it loses energy, eventually falling into the nucleus. Electrons cannot exist outside the atom in this fashion.
Note:
As an explanation for the surprising experimental results, Rutherford proposed his own physical model for subatomic structure. The atom is made up of a core charge (the current atomic nucleus), which is surrounded by a cloud of (supposedly) orbiting electrons. Rutherford did not use the term "nucleus" in his work. Rutherford only committed himself to a small centre region of very high positive or negative charge in the atom in this May \[1911\] publication.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




