
When (S)-2-bromopentane is brominated, several 2, 3-dibromopentane are formed which of the following is not formed?
(A)
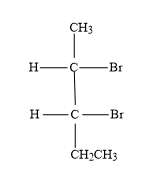
(B)
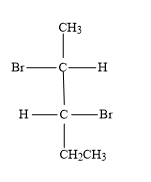
(C)
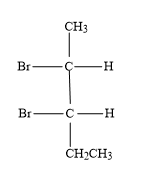
(D)



Answer
577.5k+ views
Hint: The bromination for given molecules results into formation of products with R-configuration and S-configuration as well. Thus, the feasible products according to the configuration would be formed.
Complete step by step answer:
Let us first understand the concept of chiral carbons and its configuration before actually answering the question,
Chiral carbon- A chiral or asymmetric carbon is a carbon atom that is attached to the four different types of atoms or groups of atoms.
Stereoisomerism- In stereochemistry, stereoisomerism (spatial isomerism), is a form of isomerism in which the molecules have the same molecular formula and constitution but different orientation of their atoms in space.
The stereoisomers have two types of isomers within themselves which differ in some or the other properties. They are;
1. Diastereomers
2. Enantiomers
Now, let us look forward into those:
1. Diastereomers- These are the isomers which are not the mirror images of one another. These are not related to each other by reflection.
2. Enantiomers- These are the stereoisomers which are related to each other by the reflection. They are the non-superimposable mirror images of each other. These isomers are also known as optical isomers.
R and S configuration-
The enantiomers are to be distinguished between the two non-superimposable compounds. Therefore, the absolute configuration (R and S configuration) is taken into account.
Assigning of R and S configurations consists of some rules and steps-
1. Firstly, we need to locate the central carbon atom with four different groups i.e. chiral carbon.
2. There are atoms or groups of atoms attached to the carbon having a specific atom attached directly to the chiral carbon.
We need to give the numbering to those directly attached four atoms; which decides the priority based on their respective atomic numbers. The higher the atomic number the higher the priority.
3. Afterwards, we need to draw the curved arrow moving from priority 1 to priority 2, from priority 2 to priority 3 and so on.
So, if the arrow goes clockwise then the configuration resulting will be R-configuration. Whereas, if it goes anti clockwise then it’s the S- configuration.
As we now know the basics of stereochemistry, let us see the illustration given:
When (S)-2-bromopentane is brominated the four stereoisomers of 2,3-dibromopentane are formed.
As 2,3-dibromopentane contains two chiral carbons, the possible absolute configurations are-
-(2S,3S): Second carbon (which is first chiral carbon of the molecule) will show S-configuration and third carbon (which is second chiral carbon of the molecule) will show S-configuration.
-(2R,3R): Second carbon (which is first chiral carbon of the molecule) will show R-configuration and third carbon (which is second chiral carbon of the molecule) will show R-configuration.
-(2S,3R): Second carbon (which is first chiral carbon of the molecule) will show S-configuration and third carbon (which is second chiral carbon of the molecule) will show R-configuration.
-(2R,3S): Second carbon (which is first chiral carbon of the molecule) will show R-configuration and third carbon (which is second chiral carbon of the molecule) will show S-configuration.
Thus, these are the two pairs of enantiomers which are stereoisomeric with each other.
During bromination of (S)-2-bromopentane, the configuration of second carbon (which is first chiral carbon of the molecule) is not affected. It remains as S-configuration. The configuration of the third carbon atom (which is the second chiral carbon of the molecule) can be either S or R.
Hence, the molecules with R-configuration at the second carbon atom (first chiral carbon of molecule) cannot be formed during bromination.
So, the correct answer is “Option B and C”.
Note: While checking the chirality and also while giving the priorities according to the atomic number, we should note not to make mistakes.
As, when any of the steps goes wrong, we will result in chaos due to the empirical fact that the molecular formula of every stereoisomer is the same.
Complete step by step answer:
Let us first understand the concept of chiral carbons and its configuration before actually answering the question,
Chiral carbon- A chiral or asymmetric carbon is a carbon atom that is attached to the four different types of atoms or groups of atoms.
Stereoisomerism- In stereochemistry, stereoisomerism (spatial isomerism), is a form of isomerism in which the molecules have the same molecular formula and constitution but different orientation of their atoms in space.
The stereoisomers have two types of isomers within themselves which differ in some or the other properties. They are;
1. Diastereomers
2. Enantiomers
Now, let us look forward into those:
1. Diastereomers- These are the isomers which are not the mirror images of one another. These are not related to each other by reflection.
2. Enantiomers- These are the stereoisomers which are related to each other by the reflection. They are the non-superimposable mirror images of each other. These isomers are also known as optical isomers.
R and S configuration-
The enantiomers are to be distinguished between the two non-superimposable compounds. Therefore, the absolute configuration (R and S configuration) is taken into account.
Assigning of R and S configurations consists of some rules and steps-
1. Firstly, we need to locate the central carbon atom with four different groups i.e. chiral carbon.
2. There are atoms or groups of atoms attached to the carbon having a specific atom attached directly to the chiral carbon.
We need to give the numbering to those directly attached four atoms; which decides the priority based on their respective atomic numbers. The higher the atomic number the higher the priority.
3. Afterwards, we need to draw the curved arrow moving from priority 1 to priority 2, from priority 2 to priority 3 and so on.
So, if the arrow goes clockwise then the configuration resulting will be R-configuration. Whereas, if it goes anti clockwise then it’s the S- configuration.
As we now know the basics of stereochemistry, let us see the illustration given:
When (S)-2-bromopentane is brominated the four stereoisomers of 2,3-dibromopentane are formed.
As 2,3-dibromopentane contains two chiral carbons, the possible absolute configurations are-
-(2S,3S): Second carbon (which is first chiral carbon of the molecule) will show S-configuration and third carbon (which is second chiral carbon of the molecule) will show S-configuration.
-(2R,3R): Second carbon (which is first chiral carbon of the molecule) will show R-configuration and third carbon (which is second chiral carbon of the molecule) will show R-configuration.
-(2S,3R): Second carbon (which is first chiral carbon of the molecule) will show S-configuration and third carbon (which is second chiral carbon of the molecule) will show R-configuration.
-(2R,3S): Second carbon (which is first chiral carbon of the molecule) will show R-configuration and third carbon (which is second chiral carbon of the molecule) will show S-configuration.
Thus, these are the two pairs of enantiomers which are stereoisomeric with each other.
During bromination of (S)-2-bromopentane, the configuration of second carbon (which is first chiral carbon of the molecule) is not affected. It remains as S-configuration. The configuration of the third carbon atom (which is the second chiral carbon of the molecule) can be either S or R.
Hence, the molecules with R-configuration at the second carbon atom (first chiral carbon of molecule) cannot be formed during bromination.
So, the correct answer is “Option B and C”.
Note: While checking the chirality and also while giving the priorities according to the atomic number, we should note not to make mistakes.
As, when any of the steps goes wrong, we will result in chaos due to the empirical fact that the molecular formula of every stereoisomer is the same.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




