
Starting from aniline how will you prepare benzene diazonium chloride and chlorobenzene from the later? Explain giving chemical reactions involved.
Answer
593.7k+ views
Hint: Aniline undergoes substitution reaction with nitrous acid giving benzene diazonium salt which further undergoes Sandmeyer reaction giving the chlorobenzene product.
Complete answer :
Aniline is an aromatic compound. When one hydrogen is replaced by $N{H_2}$ on a benzene ring; aniline is formed. We know the property of aromatic compounds that they are not so reactive due to resonance. But the compounds can undergo substitution reactions.
The conversion of aniline to chlorobenzene on a whole is a substitution reaction only.
In the first step, aniline is diazotized with $NaN{O_2}$ and HCl to give benzene diazonium chloride. The reaction occurs as –
First, $NaN{O_2}$ reacts with HCl giving nitrous acid. This nitrous acid reacts with aniline giving benzene diazonium salt as –
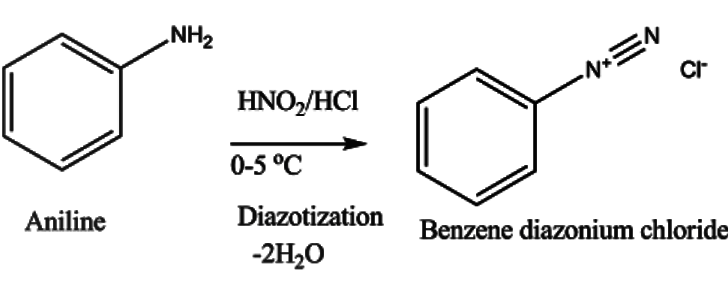
The aniline undergoes reaction with nitrous acid giving benzene diazonium chloride, NaCl and water molecules.
The benzene diazonium chloride then undergoes reaction in presence of Cu and HCl giving chlorobenzene. This reaction is known as the Sandmeyer reaction. It can be presented as -
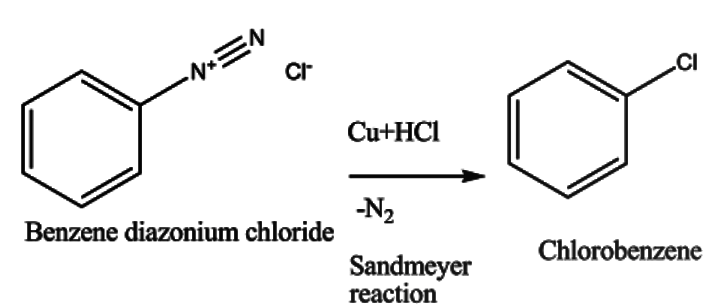
When benzene diazonium chloride undergoes reaction; Nitrogen gas is released as a byproduct which confirms the reaction.
Note :
We are normally asked to prepare aromatic compounds from aniline but not benzene because benzene is exceptionally stable and is less reactive. So, one can not easily perform any reaction with benzene.
For the preparation of benzene diazonium salt from aniline, we require cold conditions.
Complete answer :
Aniline is an aromatic compound. When one hydrogen is replaced by $N{H_2}$ on a benzene ring; aniline is formed. We know the property of aromatic compounds that they are not so reactive due to resonance. But the compounds can undergo substitution reactions.
The conversion of aniline to chlorobenzene on a whole is a substitution reaction only.
In the first step, aniline is diazotized with $NaN{O_2}$ and HCl to give benzene diazonium chloride. The reaction occurs as –
First, $NaN{O_2}$ reacts with HCl giving nitrous acid. This nitrous acid reacts with aniline giving benzene diazonium salt as –

The aniline undergoes reaction with nitrous acid giving benzene diazonium chloride, NaCl and water molecules.
The benzene diazonium chloride then undergoes reaction in presence of Cu and HCl giving chlorobenzene. This reaction is known as the Sandmeyer reaction. It can be presented as -

When benzene diazonium chloride undergoes reaction; Nitrogen gas is released as a byproduct which confirms the reaction.
Note :
We are normally asked to prepare aromatic compounds from aniline but not benzene because benzene is exceptionally stable and is less reactive. So, one can not easily perform any reaction with benzene.
For the preparation of benzene diazonium salt from aniline, we require cold conditions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




