
Structural formula of isopropyl ethanoate is:
A.
B.
C.
D.




Answer
550.8k+ views
Hint: Isopropyl methanoate is an organic compound with molecular formula ${C_4}{H_8}{O_2}$. The compound has an alternate name, isopropyl formate. It is liquid with fruity odour and is a weak base. It is a derivative of carboxylic acid ester. It is used generating flavours such as lemon, orange, strawberry and raspberry artificially.
Complete step by step answer:
The compound isopropyl ethanoate belongs to the category of organic compounds known as carboxylic acid esters, in which carbon atom from carbonyl group is attached to alkyl or aryl group through an oxygen atom and forms an ester. These compounds are considered as the derivatives of carboxylic groups.
Let us try to understand the structure of compounds given in each option and name them in order to find the correct answer.
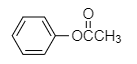
In the option (A), the structure of compound given is shown below.
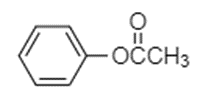
The compound shown above is an ester in which the carbon atom of carbonyl group is attached to phenyl group through an oxygen atom, forming an ester. The ester part contains two carbon atoms including one from methyl group and one carbonyl carbon. Hence, the name is phenyl acetate. Therefore, option (A) is incorrect.
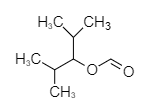
In option (B), the structure of compound given is shown below.
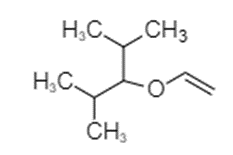
The compound shown above contains a carbon atom of carbonyl group attached to a substituted alkyl group through an oxygen atom, forming an ester. It is a derivative of methanoate and the oxygen atom of methanoate is attached to the substituted alkyl group. The correct name of the compound is 2,4-dimethyl-3-yl formate. Therefore, option (B) is incorrect.
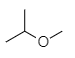
In the option (C), the structure of compound given is shown below.
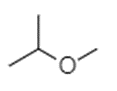
The compound shown above contains no carbonyl group but only oxygen atom attached to two different alkyl groups from both sides. This compound is not an ester but it is an ether. The name of the compound is 2-methoxypropane. Therefore, option (C) is incorrect.
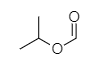
In the option (D), the structure of compound given is shown below.
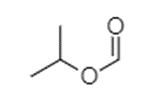
The compound shown above contains a carbon atom of carbonyl group attached to a propyl group through an oxygen atom, forming an ester. The ester part here is formate or methanoate due to the presence of only one carbon atom in it that is carbonyl carbon. The second oxygen of formate is attached to a propyl group at second carbon so this is an isopropyl group. The name of the compound is isopropyl methanoate. It is an ester of formic acid with isopropanol.
So, the correct answer is Option D.
Additional information:
Esters are one of the important classes of organic compounds and generally they are represented as $RCOOR'$, where R and R’ both are alkyl groups. The commonly used method of preparing esters involves reaction between carboxylic acid with alcohol. Most of the naturally occurring fats and oils are derivatives of esters. Due are fragrant compounds and hence, found in essential oils too.
Note: While naming the ester, you should remember following things.
An ester is made up of two parts, an alcohol and a carboxylic acid.
While naming an ester, the first step is to identify the oxygen that is bonded to the carbon atom from both sides that is carbonyl carbon from one side and alkyl or aryl carbon from the other side.
Next step is to start numbering the carbon chains from both sides of the oxygen atom.
The name of the carbonyl side of oxygen will be of an alkane whereas the other side of oxygen will be named as alkyl group.
Complete step by step answer:
The compound isopropyl ethanoate belongs to the category of organic compounds known as carboxylic acid esters, in which carbon atom from carbonyl group is attached to alkyl or aryl group through an oxygen atom and forms an ester. These compounds are considered as the derivatives of carboxylic groups.
Let us try to understand the structure of compounds given in each option and name them in order to find the correct answer.
In the option (A), the structure of compound given is shown below.

The compound shown above is an ester in which the carbon atom of carbonyl group is attached to phenyl group through an oxygen atom, forming an ester. The ester part contains two carbon atoms including one from methyl group and one carbonyl carbon. Hence, the name is phenyl acetate. Therefore, option (A) is incorrect.
In option (B), the structure of compound given is shown below.

The compound shown above contains a carbon atom of carbonyl group attached to a substituted alkyl group through an oxygen atom, forming an ester. It is a derivative of methanoate and the oxygen atom of methanoate is attached to the substituted alkyl group. The correct name of the compound is 2,4-dimethyl-3-yl formate. Therefore, option (B) is incorrect.
In the option (C), the structure of compound given is shown below.

The compound shown above contains no carbonyl group but only oxygen atom attached to two different alkyl groups from both sides. This compound is not an ester but it is an ether. The name of the compound is 2-methoxypropane. Therefore, option (C) is incorrect.
In the option (D), the structure of compound given is shown below.

The compound shown above contains a carbon atom of carbonyl group attached to a propyl group through an oxygen atom, forming an ester. The ester part here is formate or methanoate due to the presence of only one carbon atom in it that is carbonyl carbon. The second oxygen of formate is attached to a propyl group at second carbon so this is an isopropyl group. The name of the compound is isopropyl methanoate. It is an ester of formic acid with isopropanol.
So, the correct answer is Option D.
Additional information:
Esters are one of the important classes of organic compounds and generally they are represented as $RCOOR'$, where R and R’ both are alkyl groups. The commonly used method of preparing esters involves reaction between carboxylic acid with alcohol. Most of the naturally occurring fats and oils are derivatives of esters. Due are fragrant compounds and hence, found in essential oils too.
Note: While naming the ester, you should remember following things.
An ester is made up of two parts, an alcohol and a carboxylic acid.
While naming an ester, the first step is to identify the oxygen that is bonded to the carbon atom from both sides that is carbonyl carbon from one side and alkyl or aryl carbon from the other side.
Next step is to start numbering the carbon chains from both sides of the oxygen atom.
The name of the carbonyl side of oxygen will be of an alkane whereas the other side of oxygen will be named as alkyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




