
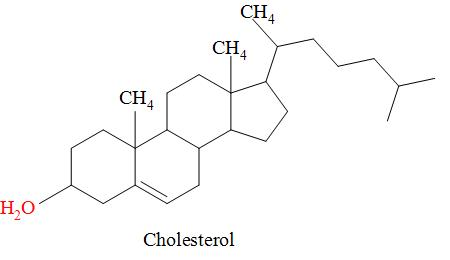
The diagram shows the structure of cholesterol.
Which statements about cholesterol are correct?
1. The molecule contains a secondary alcohol group.
2. the molecule contains two$\pi $ bonds.
3. all carbon atoms in the four rings lie in the same plane.
(A) 1,2 and 3 are correct
(B) 1 and 2 are correct
(C) 2 and 3 are correct
(D)1 only is correct

Answer
584.4k+ views
Hint: Cholesterol is tetracyclic alcohol in which the alcohol is attached to the secondary carbon atom to that cyclic ring which is in the plane and it consists of only one double bond . Now identify the correct statement.
Complete step by step answer:
Cholesterol is an organic compound of a long chain of fatty acids and is a type of steroid ( these are present in the human body naturally and just like the hormones inside our body and it helps to reduce the inflammation i.e. lowers the swelling and the redness.) its molecular formula is ${{\text{C}}_{27}}{{\text{H}}_{46}}\text{O}$.
The structure of cholesterol is a tetracyclic alcohol i.e. it consists of four cyclic rings and the alcohol i.e. the -OH group is attached to that cyclic ring which is in the plane and is attached at C-3 and is secondary alcoholic group because the -OH group is attached to the secondary carbon atom and that ring is fused with the other three rings.
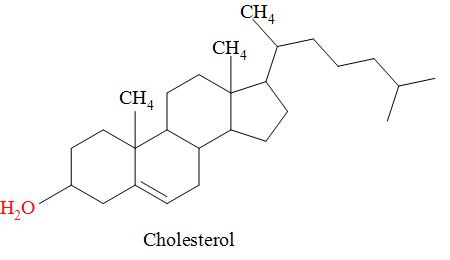
The four cyclic rings are not lying in the same plane i.e. they all occupy different planes and from the structure of cholesterol it is clear that it consists of only double bonds i.e. it means that there is one pi bond in the cholesterol.
So, thus from all the above given statements only statement 1. Is correct and rest all others are incorrect.
Hence, the option(D) is correct.
Note: Its functions are that it acts as a building block for the human body and helps in the production of bile too in the liver and for vitamin D too and thus, it is very essential and plays an important role in our body.
Complete step by step answer:
Cholesterol is an organic compound of a long chain of fatty acids and is a type of steroid ( these are present in the human body naturally and just like the hormones inside our body and it helps to reduce the inflammation i.e. lowers the swelling and the redness.) its molecular formula is ${{\text{C}}_{27}}{{\text{H}}_{46}}\text{O}$.
The structure of cholesterol is a tetracyclic alcohol i.e. it consists of four cyclic rings and the alcohol i.e. the -OH group is attached to that cyclic ring which is in the plane and is attached at C-3 and is secondary alcoholic group because the -OH group is attached to the secondary carbon atom and that ring is fused with the other three rings.

The four cyclic rings are not lying in the same plane i.e. they all occupy different planes and from the structure of cholesterol it is clear that it consists of only double bonds i.e. it means that there is one pi bond in the cholesterol.
So, thus from all the above given statements only statement 1. Is correct and rest all others are incorrect.
Hence, the option(D) is correct.
Note: Its functions are that it acts as a building block for the human body and helps in the production of bile too in the liver and for vitamin D too and thus, it is very essential and plays an important role in our body.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




