
The nitride ion in lithium nitride is composed of:
A. 7 protons and 7 electrons
B. 10 protons and 7 electrons
C. 7 protons and 10 electrons
D. 10 protons and 10 electrons
Answer
232.8k+ views
Hint: Lithium nitride is a compound with chemical formula $L{i_3}N$. It is the only stable alkali metal nitride. It reacts with water to make lithium hydroxide and ammonia. Further, electrons are a type of subatomic particle with a negative charge and protons are a type of subatomic particle with a positive charge.
Complete step by step answer:
Lithium nitride is a solid which has a reddish-pink color and a high melting point. Its chemical formula is $L{i_3}N$. Its structure is as shown:
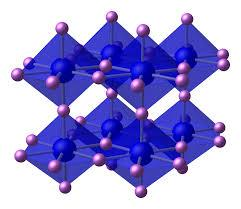
The nitride ion in this compound is ${N^{3 - }}$ which has a charge of – 3. Since, the atomic number of nitrogen is 7, so the number of protons is also 7 because the number of protons in the nucleus of the atom is equal to the atomic number (Z).
Now, the number of electrons will be 10 because the number of protons were 7 and further, 3 more electrons were added. (Because there is a charge of – 3 on the nitride ion).
So, there were 7+3=10 electrons.
Hence, option C is correct.
Note:
The nitrides of boron, vanadium, silicon, titanium and tantalum are very refractory, resistant to chemical attack, and hard and thus are useful as abrasives and in making crucibles. Lithium nitride is used to store hydrogen and is also used as a source of the nitride ion. Moreover, nitride compounds are also used as insulators
Complete step by step answer:
Lithium nitride is a solid which has a reddish-pink color and a high melting point. Its chemical formula is $L{i_3}N$. Its structure is as shown:

The nitride ion in this compound is ${N^{3 - }}$ which has a charge of – 3. Since, the atomic number of nitrogen is 7, so the number of protons is also 7 because the number of protons in the nucleus of the atom is equal to the atomic number (Z).
Now, the number of electrons will be 10 because the number of protons were 7 and further, 3 more electrons were added. (Because there is a charge of – 3 on the nitride ion).
So, there were 7+3=10 electrons.
Hence, option C is correct.
Note:
The nitrides of boron, vanadium, silicon, titanium and tantalum are very refractory, resistant to chemical attack, and hard and thus are useful as abrasives and in making crucibles. Lithium nitride is used to store hydrogen and is also used as a source of the nitride ion. Moreover, nitride compounds are also used as insulators
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




