
The orbital picture of singlet carbene $\left( {:C{H_2}} \right)$ can be drawn as:
(A).

(B).
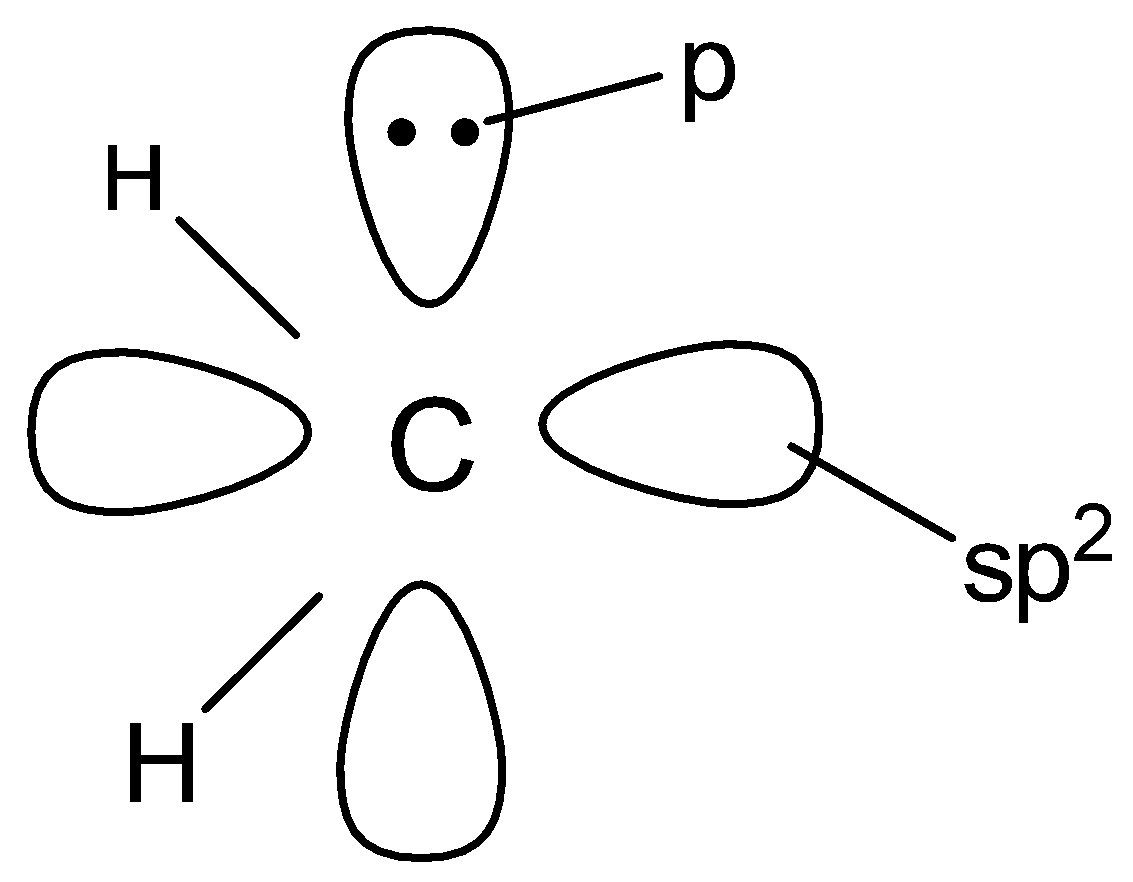
(C).

(D). None of these.



Answer
576k+ views
Hint: Neutral divalent carbon species in which the carbon atom is bonded to two monovalent atoms or groups and also contains two non- bonding electrons are called carbenes .
Complete answer:
Carbenes are generally produced either by photolysis ( irradiation with UV light ) or thermolysis or pyrolysis ( action of heat ) of diazoalkanes or ketenes .
Now talking about the orbital structure of carbene , there are two types of carbenes , that is singlet carbene and triplet carbene . Singlet carbenes are less stable than triplet carbenes since triplet carbenes have a linear structure and behave as a diradical whereas singlet carbenes have bent structure .
In singlet carbenes , the central carbon atom is $s{p^2}$ hybridised . Two of the $s{p^2}$ - hybridised orbitals form two $\sigma $ - bonds with two monovalent atoms or groups while the third $s{p^2}$ - hybridised orbital contains two non - bonding electrons . The unhybridized p - orbital is , however, empty . Thus , a singlet carbene has a bent structure .
So according to the above description , the orbital structure of carbene will be

Hence option A is correct .
Note:
Just like carbocations , carbenes are short lived highly reactive chemical species since the central carbon atom has only six electrons in its valence shell and thus has a strong tendency to complete its octet by gaining two more electrons . Carbenes , thus behave as Lewis acids or electrophiles .
Complete answer:
Carbenes are generally produced either by photolysis ( irradiation with UV light ) or thermolysis or pyrolysis ( action of heat ) of diazoalkanes or ketenes .
Now talking about the orbital structure of carbene , there are two types of carbenes , that is singlet carbene and triplet carbene . Singlet carbenes are less stable than triplet carbenes since triplet carbenes have a linear structure and behave as a diradical whereas singlet carbenes have bent structure .
In singlet carbenes , the central carbon atom is $s{p^2}$ hybridised . Two of the $s{p^2}$ - hybridised orbitals form two $\sigma $ - bonds with two monovalent atoms or groups while the third $s{p^2}$ - hybridised orbital contains two non - bonding electrons . The unhybridized p - orbital is , however, empty . Thus , a singlet carbene has a bent structure .
So according to the above description , the orbital structure of carbene will be

Hence option A is correct .
Note:
Just like carbocations , carbenes are short lived highly reactive chemical species since the central carbon atom has only six electrons in its valence shell and thus has a strong tendency to complete its octet by gaining two more electrons . Carbenes , thus behave as Lewis acids or electrophiles .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




